Abstract
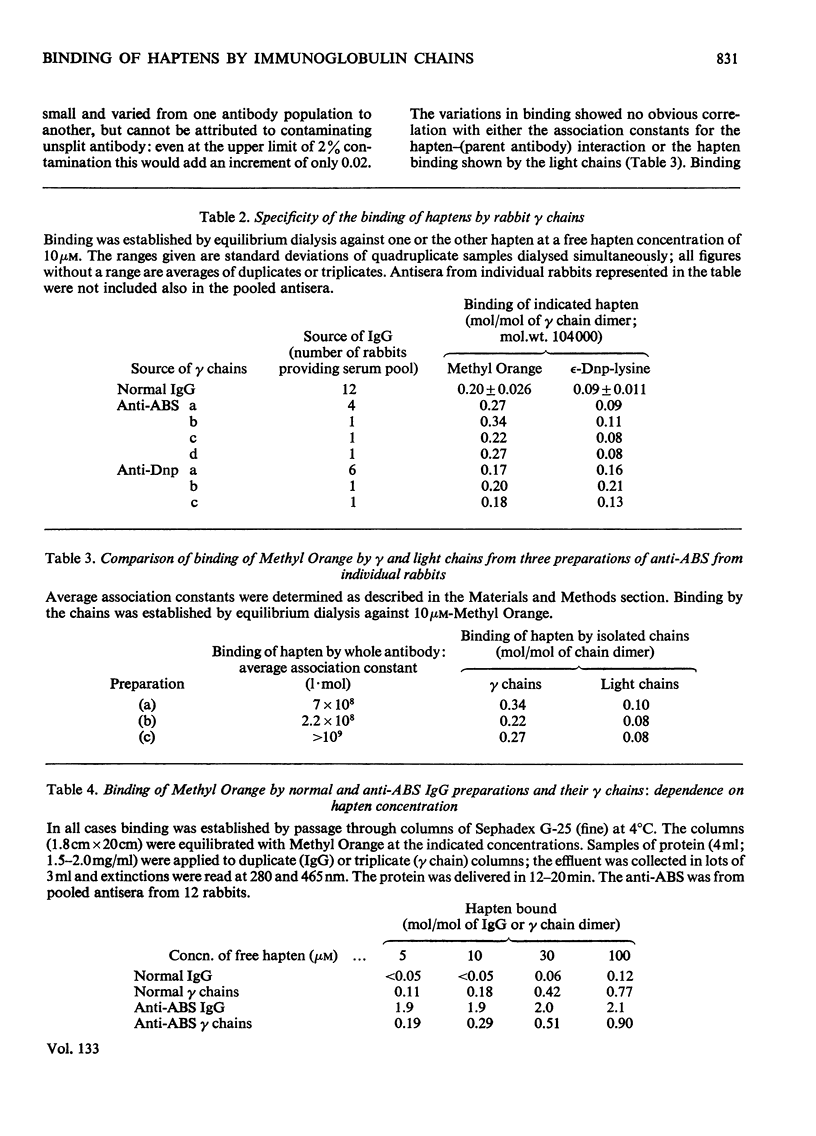
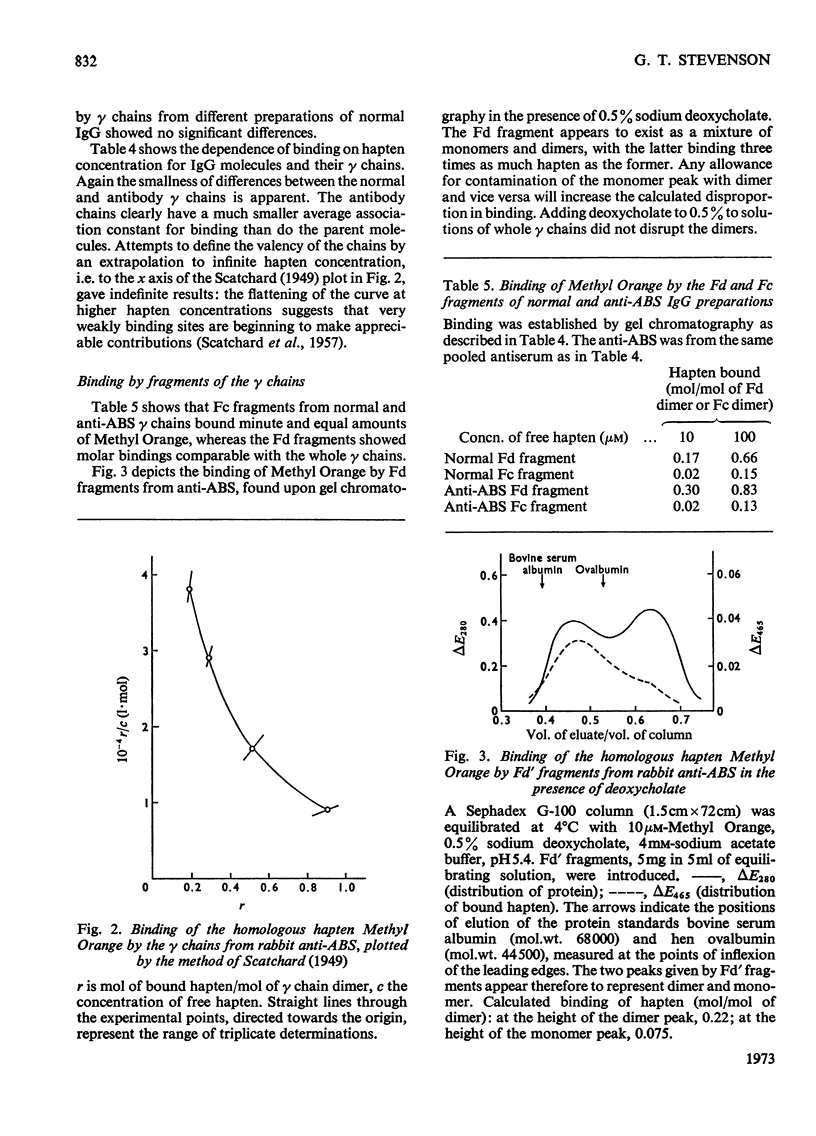
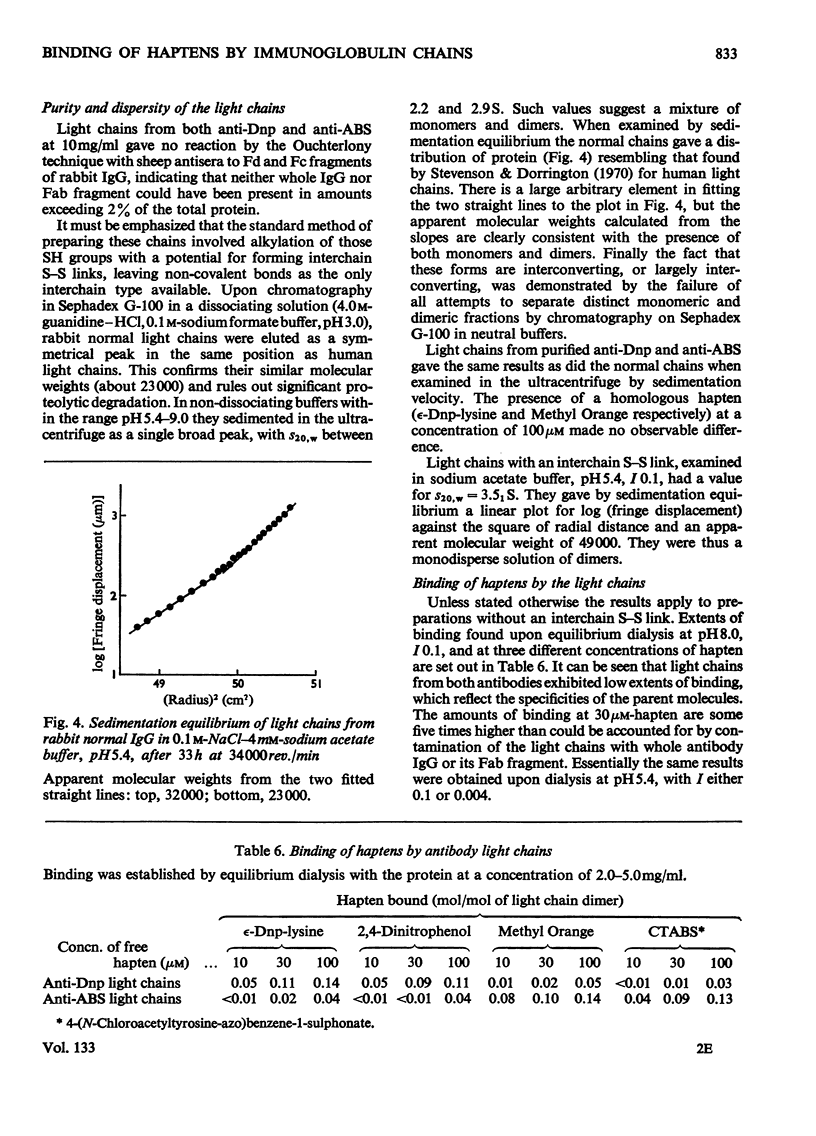
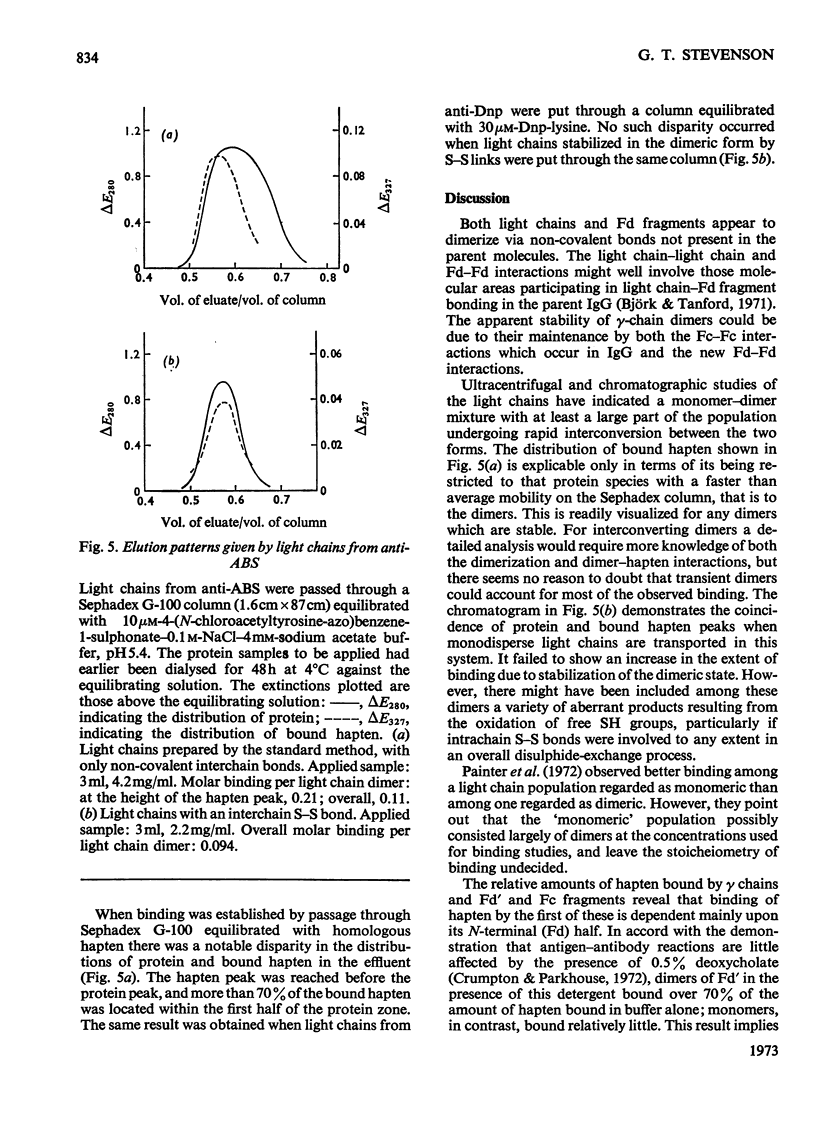
1. The binding of haptens by the polypeptide chains derived from two rabbit immunoglobulin G antibodies was examined by gel chromatography and equilibrium dialysis. 2. The γ chains were examined in a dilute sodium acetate buffer, pH5.4, in which they exist as a monodisperse solution of dimers; aggregation of the protein promoted by some haptens had to be avoided. These chains exhibited variable extents of binding, reflecting the specificities of the parent antibody molecules, usually with only small increments above the binding by γ chains from normal immunoglobulin G. 3. The light chains existed as an interconverting mixture of monomers and dimers in all buffers of near neutral pH that were examined. They bound small amounts of hapten, again broadly reflecting the specificities of the parent antibody molecules. 4. For both the γ and light chains the dimeric state appeared necessary for appreciable binding of hapten. Apparently in each case the partners in the dimer interact in a manner analogous to the γ chain–light chain interaction in the parent antibody molecule, to give a site analogous to the antibody site. This implies that the binding of antigens by isolated chains has a large fortuitous element, providing no reliable indication of their contributions to the original antibody sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk I., Tanford C. Gross conformation of free polypeptide chains from rabbit immunoglobulin G. II. Light chain. Biochemistry. 1971 Apr 13;10(8):1280–1288. doi: 10.1021/bi00784a002. [DOI] [PubMed] [Google Scholar]
- Cohen S., Milstein C. Structure and biological properties of immunoglobulins. Adv Immunol. 1967;7:1–89. doi: 10.1016/s0065-2776(08)60126-1. [DOI] [PubMed] [Google Scholar]
- Crone M., Koch C., Simonsen M. The elusive T cell receptor. Transplant Rev. 1972;10:36–56. doi: 10.1111/j.1600-065x.1972.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Parkhouse R. M.E. Comparison of the effects of various detergents on antigen-antibody interaction. FEBS Lett. 1972 May 1;22(2):210–212. doi: 10.1016/0014-5793(72)80047-4. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman J. B. Immunoglobulins. Annu Rev Biochem. 1966;35:835–872. doi: 10.1146/annurev.bi.35.070166.004155. [DOI] [PubMed] [Google Scholar]
- GALLY J. A., EDELMAN G. M. PROTEIN-PROTEIN INTERACTIONS AMONG L POLYPEPTIDE CHAINS OF BENCE-JONES PROTEINS AND HUMAN GAMMA-GLOBULINS. J Exp Med. 1964 May 1;119:817–836. doi: 10.1084/jem.119.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. On the prevalence of "nonspecific" binding at the specific binding sites of globular proteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1057–1063. doi: 10.1073/pnas.65.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. W., Donch J. J. Phage-neutralizing activity in light polypeptide chains of rabbit antibody. Immunochemistry. 1965 Dec;2(4):351–357. doi: 10.1016/0019-2791(65)90035-2. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Inhibition of human mixed lymphocyte reaction by antibodies to immunoglobulin light chain determinants. Clin Exp Immunol. 1971 Sep;9(3):313–328. [PMC free article] [PubMed] [Google Scholar]
- Jaton J. C., Klinman N. R., Givol D., Sela M. Recovery of antibody activity upon reoxidation of completely reduced polyalanyl heavy chain and its Fd fragment derived from anti-2,4-dinitrophenyl antibody. Biochemistry. 1968 Dec;7(12):4185–4195. doi: 10.1021/bi00852a008. [DOI] [PubMed] [Google Scholar]
- METZGER H., SINGER S. J. BINDING CAPACITY OF REDUCTIVELY FRAGMENTED ANTIBODIES TO THE 2,4-DINITROPHENYL GROUP. Science. 1963 Nov 8;142(3593):674–675. doi: 10.1126/science.142.3593.674. [DOI] [PubMed] [Google Scholar]
- Mangalo R., Raynaud M. Activité anticorps dans des préparations de chaines légères. Ann Inst Pasteur (Paris) 1967 Oct;113(4):549–567. [PubMed] [Google Scholar]
- Mason S., Warner N. L. The immunoglobulin nature of the antigen recognition site on cells mediating transplantation immunity and delayed hypersentivity. J Immunol. 1970 Mar;104(3):762–765. [PubMed] [Google Scholar]
- Milstein C., Pink J. R. Structure and evolution of immunoglobulins. Prog Biophys Mol Biol. 1970;21:209–263. doi: 10.1016/0079-6107(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Dorrington K. J., Rockey J. H. Equine antihapten antibody. The molecular weights of the subunits of equine immunoglobulins. Biochemistry. 1969 Mar;8(3):1247–1258. doi: 10.1021/bi00831a060. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Sage H. J., Tanford C. Contributions of heavy and light chains of rabbit immunoglobulin G to antibody activity. I. Binding studies on isolated heavy and light chains. Biochemistry. 1972 Apr 11;11(8):1327–1337. doi: 10.1021/bi00758a001. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Osterland C. K. Hydrophobic binding sites on immunoglobulins. Biochemistry. 1970 Mar 3;9(5):1074–1082. doi: 10.1021/bi00807a004. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Warner N. L. Suppression of graft versus host reactions in chickens by pretreatment of leucocytes with anti-light chain sera. Cell Immunol. 1972 Mar;3(3):470–477. doi: 10.1016/0008-8749(72)90252-3. [DOI] [PubMed] [Google Scholar]
- STEVENSON G. T. Further studies of the gamma-related proteins of normal urine. J Clin Invest. 1962 May;41:1190–1198. doi: 10.1172/JCI104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. T., Dorrington K. J. The recombination of dimers of immunoglobulin peptide chains. Biochem J. 1970 Aug;118(5):703–712. doi: 10.1042/bj1180703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe N. O., Singer S. J. The affinity-labeled residues in antibody active sites. II. Nearest-neighbor analyses. Biochemistry. 1969 Nov;8(11):4523–4534. doi: 10.1021/bi00839a045. [DOI] [PubMed] [Google Scholar]
- Turner M. W., Rowe D. S. Antibodies of IgA and IgG class in normal human urine. Immunology. 1967 Jun;12(6):689–699. [PMC free article] [PubMed] [Google Scholar]
- UTSUMI S., KARUSH F. THE SUBUNITS OF PURIFIED RABBIT ANTIBODY. Biochemistry. 1964 Sep;3:1329–1338. doi: 10.1021/bi00897a024. [DOI] [PubMed] [Google Scholar]
- Vaughan J. H., Jacox R. F., Gray B. A. Light and heavy chain components of gamma globulins in urines of normal persons and patients with agammaglobulinemia. J Clin Invest. 1967 Feb;46(2):266–279. doi: 10.1172/JCI105529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yoo T. J., Roholt O. A., Pressman D. Specific binding activity of isolated light chains of antibodies. Science. 1967 Aug 11;157(3789):707–709. doi: 10.1126/science.157.3789.707. [DOI] [PubMed] [Google Scholar]



