Abstract
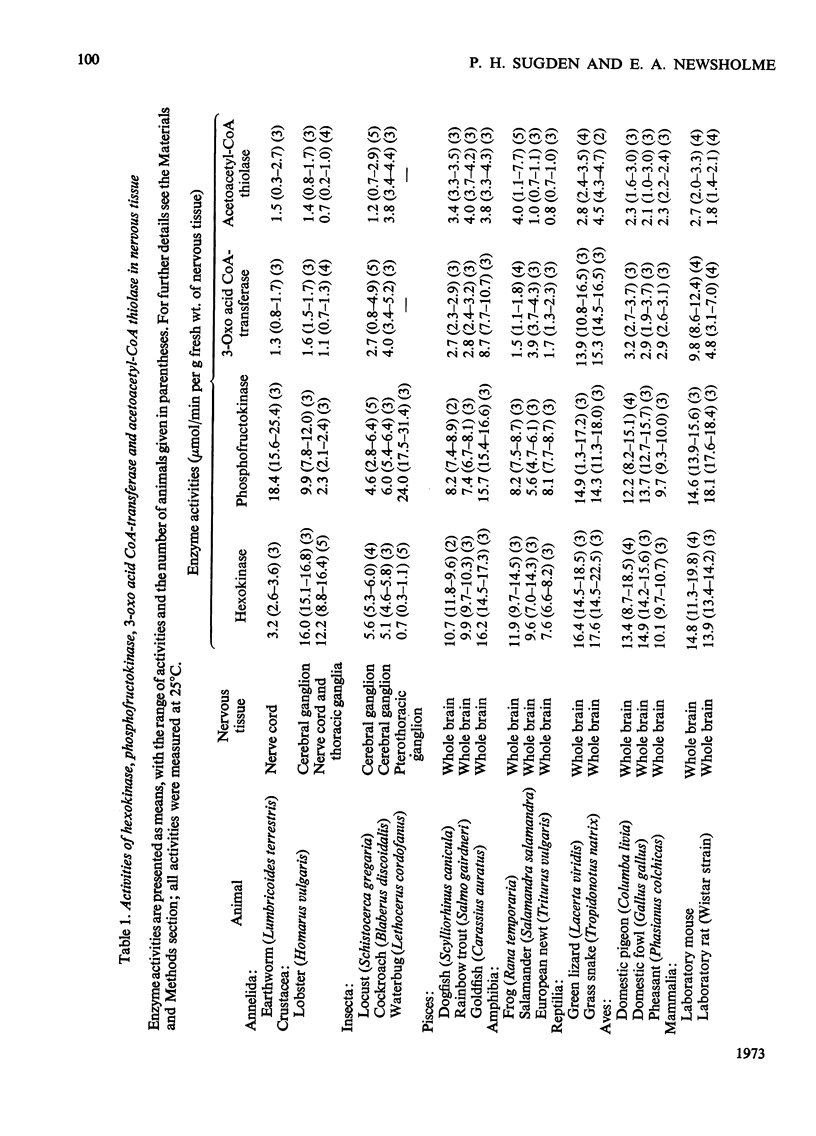
1. The maximum activities of hexokinase and phosphofructokinase in nervous tissue from 18 different animals from different phyla range from 5.1 to 17.6 and from 24.0μmol/min per g fresh wt. respectively. In any one tissue the activities of these two enzymes are, in general, very similar. The rate of glucose utilization by the brain in vivo is much lower than the activities of hexokinase or phosphofructokinase. It is suggested that the high activities of these enzymes indicate a capacity for glycolysis which may be used by the brain during hypoxia or during conditions of extreme neuronal activity. 2. The activities of 3-oxo acid CoA-transferase and acetoacetyl-CoA thiolase in the nervous tissues range from 1.1 to 15.3 and from 0.7 to 4.5μmol/min per g fresh wt. respectively. Unfortunately the activities of these enzymes cannot be used to estimate maximal flux through the ketone-body-utilization pathway, since they may catalyse reactions that are close to equilibrium. Nonetheless, the presence of these enzymes in nervous tissue from a large variety of animals suggests that the importance of ketone bodies as a fuel for nervous tissue may be widespread in the animal kingdom.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Metabolic aspects of enzyme activity regulation. Symp Soc Exp Biol. 1973;27:429–460. [PubMed] [Google Scholar]
- Newsholme E. A., Sugden P. H. The apparent inhibition of phosphofrunctokinase by reduced nicotinamide-adenine dinucleotide: a problem of coupled-enzyme assays. Biochem J. 1970 Oct;119(4):787–789. doi: 10.1042/bj1190787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Taylor K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J. 1969 May;112(4):465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Effects of fatty acids, ketone bodies, lactate and pyruvate on glucose utilization by guinea-pig cerebral cortex slices. Biochem J. 1967 Aug;104(2):519–523. doi: 10.1042/bj1040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]


