Abstract
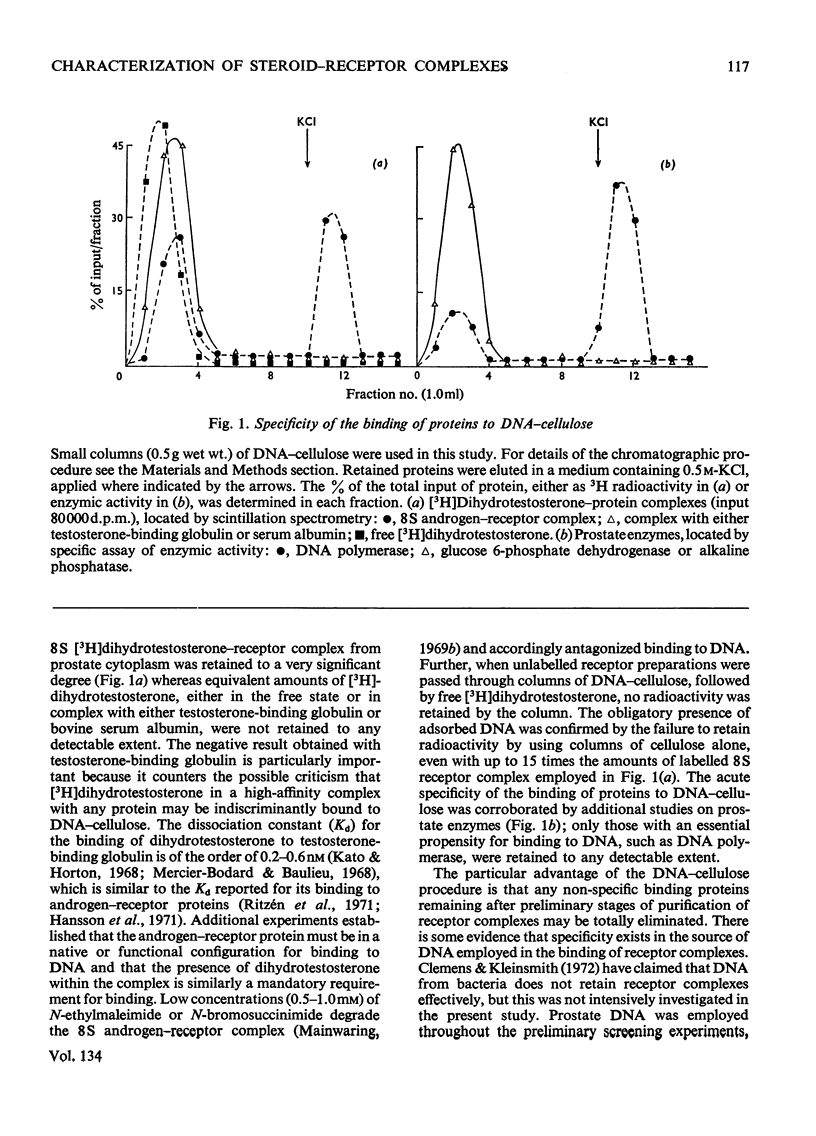
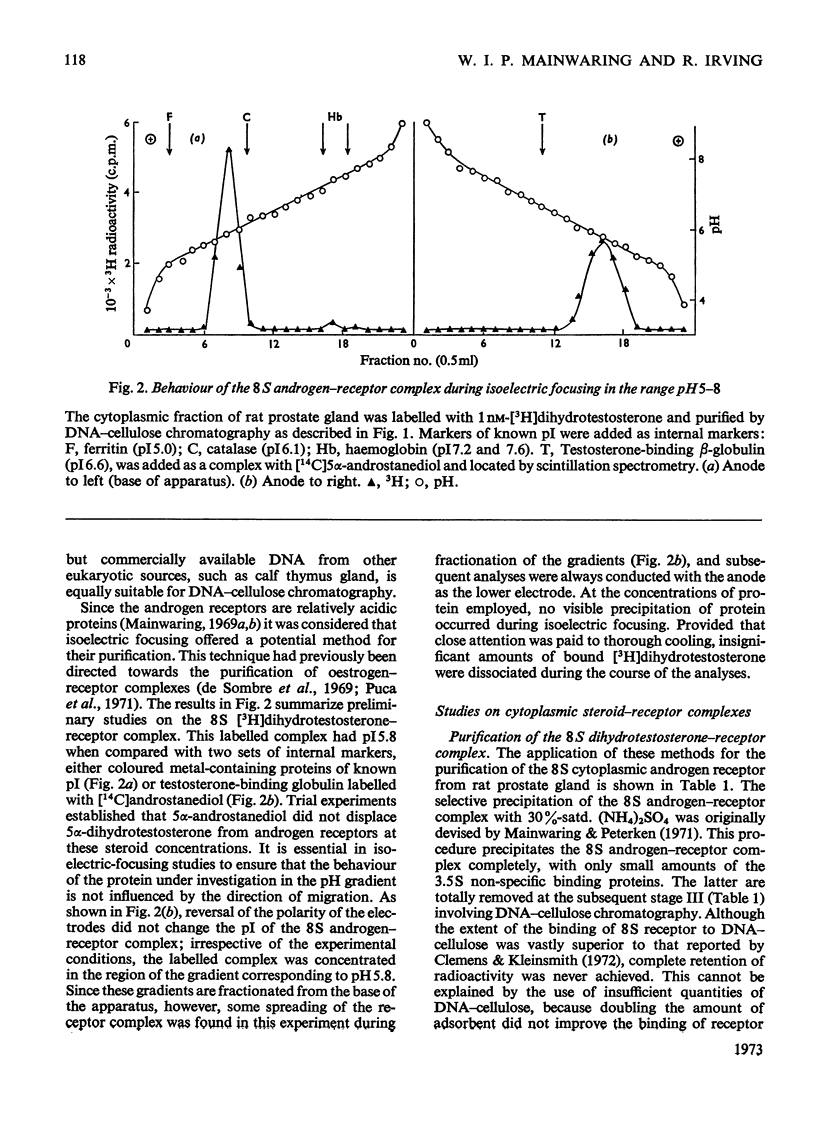
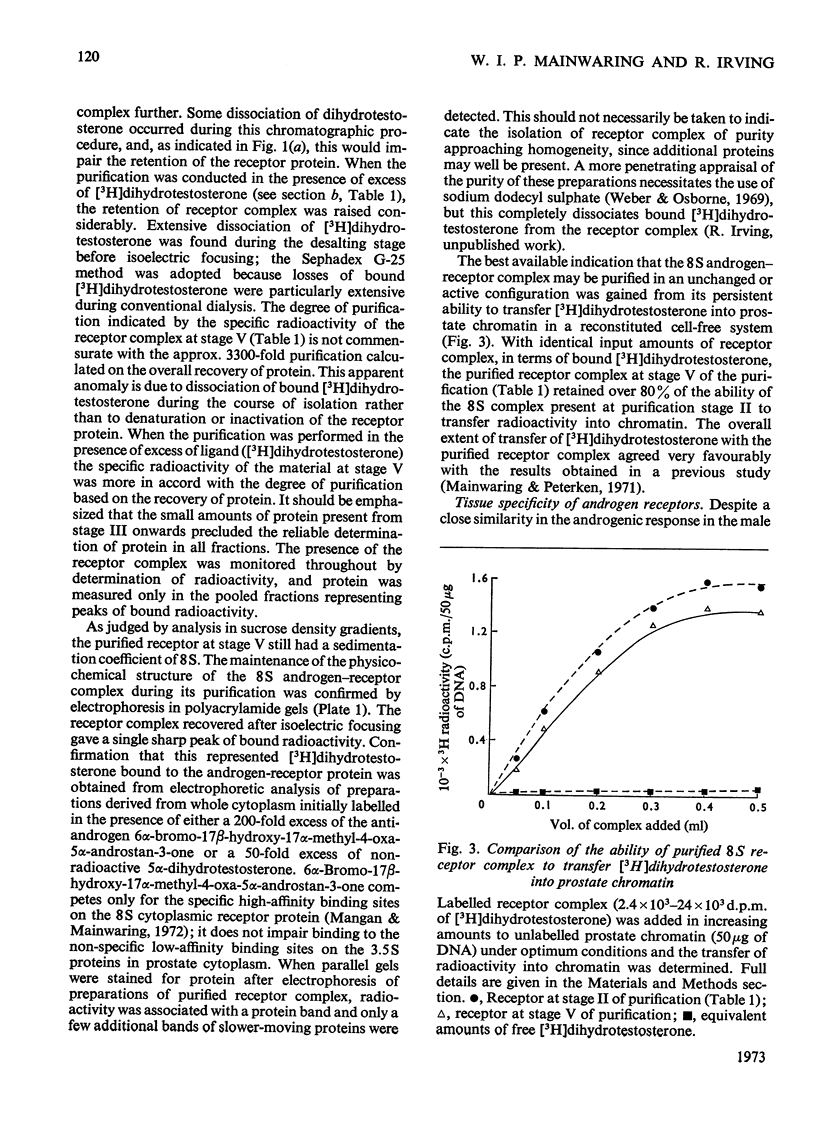
1. Two characteristic properties of the specific high-affinity steroid-binding proteins or receptors, their ability to bind to DNA–cellulose and their relatively acidic isoelectric point, have been exploited as a means of purification. These two fundamental properties distinguish the receptors from the steroid-binding proteins in serum and the non-specific low-affinity steroid-binding proteins in hormone-responsive cells. 2. A significant degree of purification of both cytoplasmic and nuclear steroid–receptor complexes can be achieved with practical facility by these procedures. The purity of the receptor complexes is sufficient to enable studies on their possible control of metabolic processes to be investigated in the future. 3. After extensive purification the physicochemical properties of the cytoplasmic androgen–receptor complex, such as sedimentation coefficient, were unchanged. Further, the purified complex fully retained at least one of its fundamental physiological properties, namely the ability to transfer 5α-dihydrotestosterone (17β-hydroxy-5α-androstan-3-one) into chromatin in vitro. 4. The methods may also be employed for studying the changes in the structure and properties of the receptor complexes that are an essential prerequisite for the transfer of cytoplasmic receptor complexes into nuclear chromatin. The temperature-dependence of the binding of androgen–receptor complexes into chromatin is essentially due to a major change in cytoplasmic receptor complex before its attachment to nuclear chromatin. 5. The resolution of these analytical procedures was sufficient to enable a critical comparison of the receptor proteins from different male accessory glands to be undertaken. From these studies, no substantial evidence in support of the tissue specificity of androgen receptors could be established; rather the receptors from different androgen-dependent glands were remarkably similar in physicochemical properties. 6. Although the methods were initially developed for the partial purification of androgen–receptor complexes, they are equally suitable for the prompt and extensive purification of oestrogen–receptor and progesterone–receptor complexes.
Full text
PDF




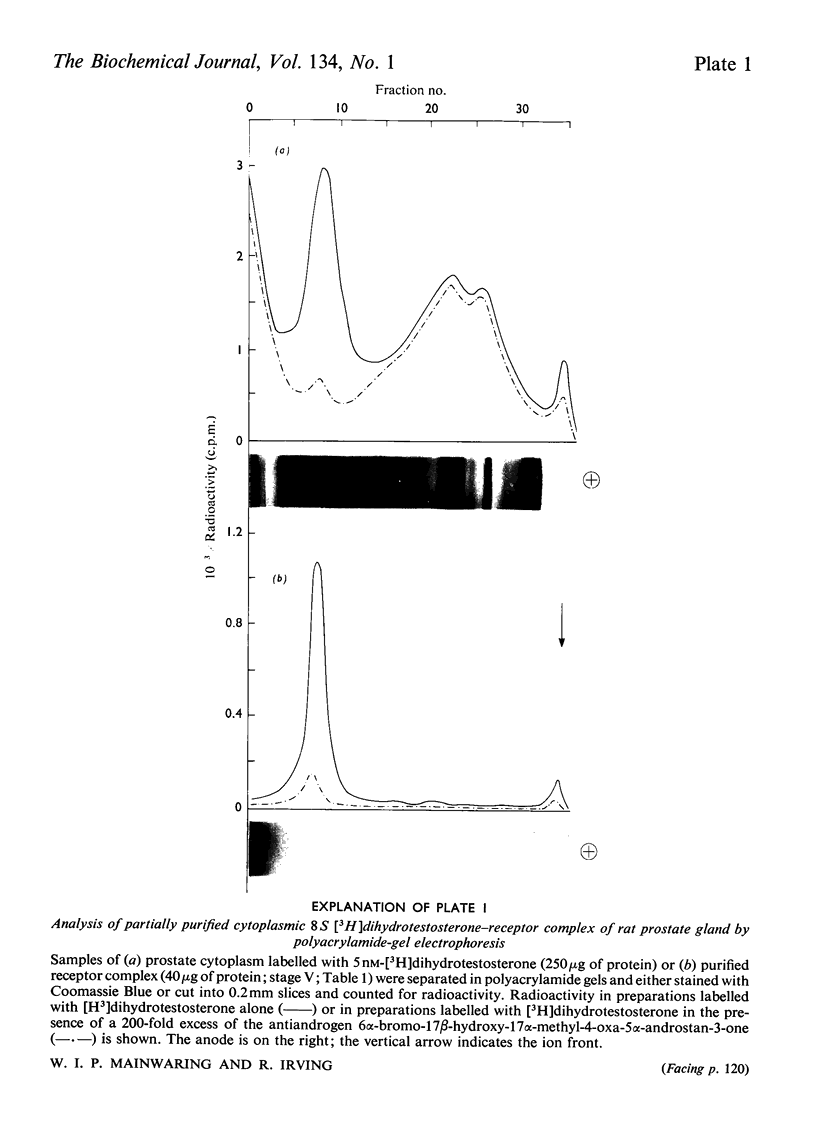
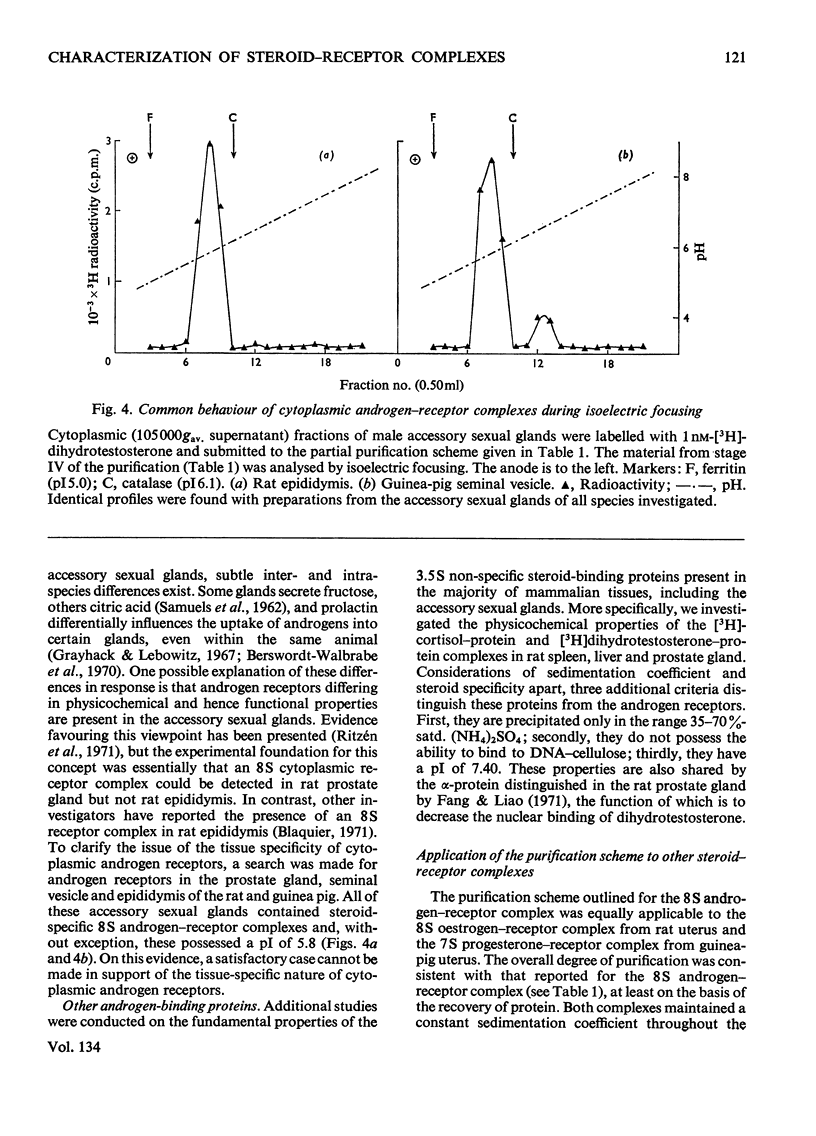
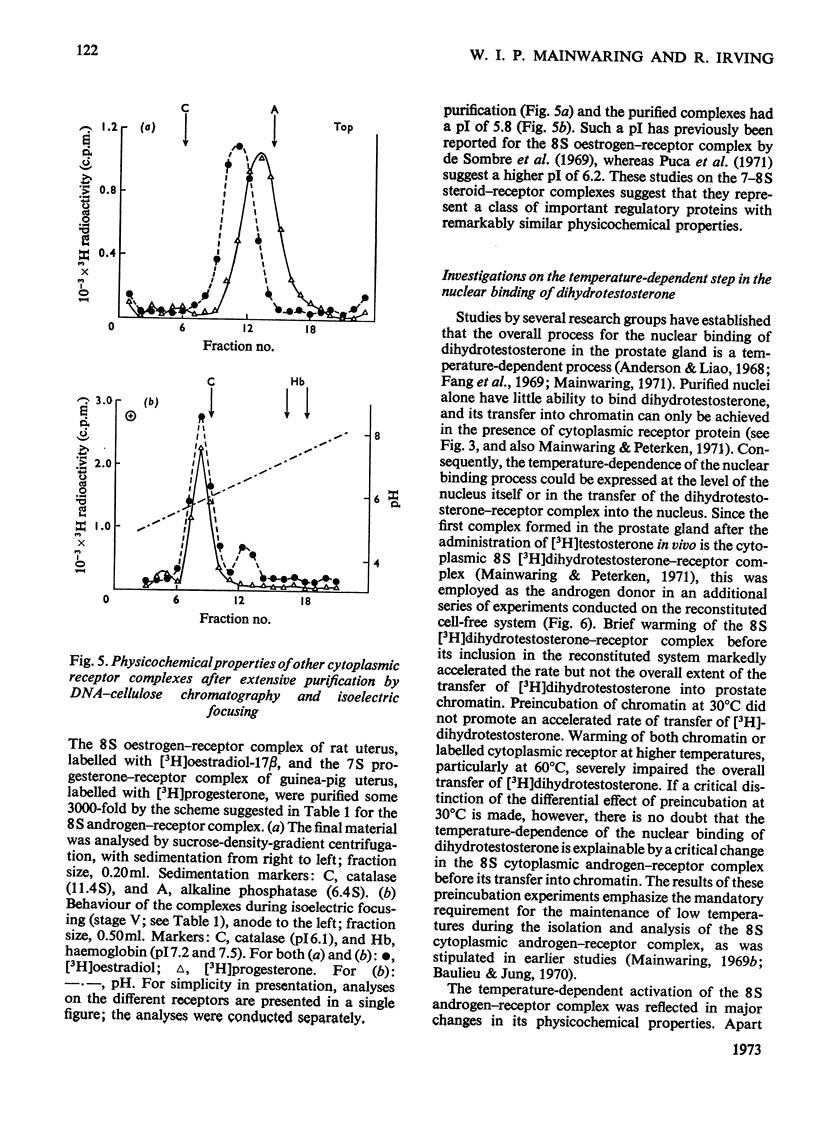
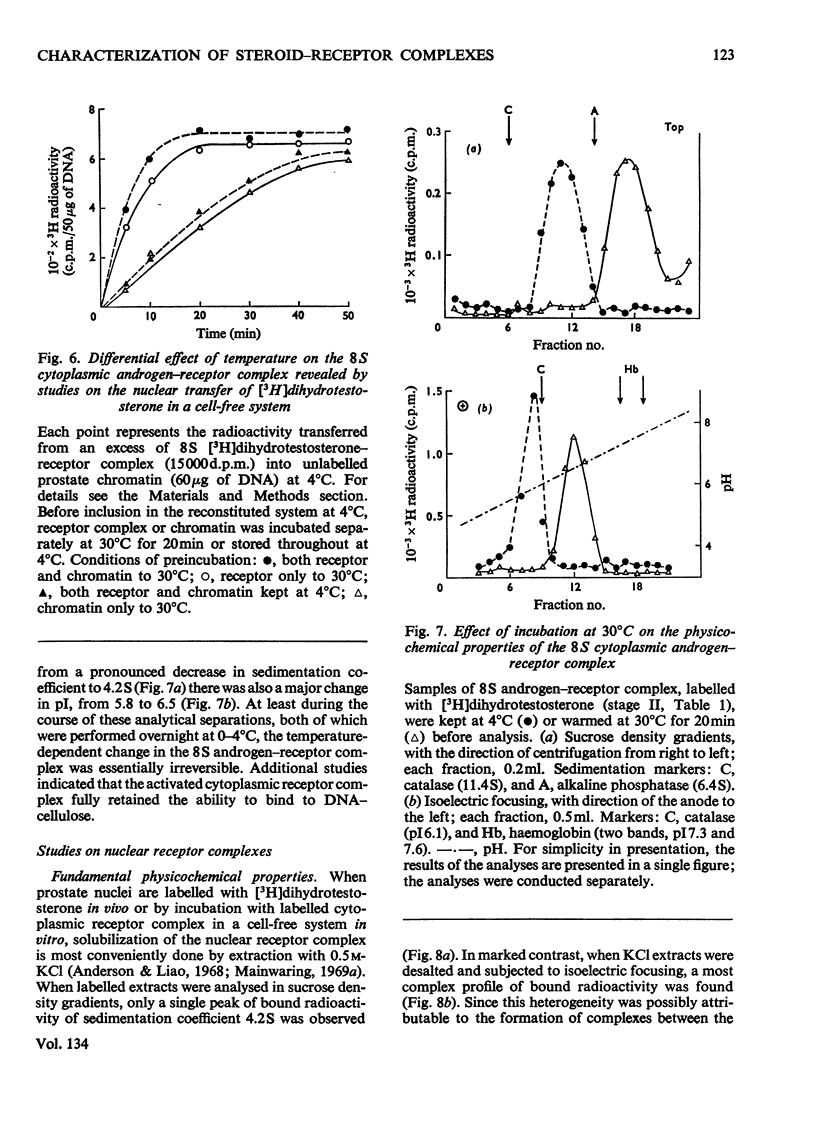



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Anderson K. M., Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968 Jul 20;219(5151):277–279. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E. E., Jung I. A prostatic cytosol receptor. Biochem Biophys Res Commun. 1970 Feb 20;38(4):599–606. doi: 10.1016/0006-291x(70)90623-6. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belham J. E., Neal G. E. Testosterone action in the rat ventral prostate. The effects of diethylstilboestrol and cyproterone acetate on the metabolism of ( 3 H)testosterone and the retention of labelled metabolites by rat ventral prostate in vivo and in vitro. Biochem J. 1971 Nov;125(1):81–91. doi: 10.1042/bj1250081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berswordt-Wallrabe R V. O., Bielitz U., Elger W., Steinbeck H. Progesterone and the ventral prostate gland in juvenile rats. J Urol. 1970 Feb;103(2):180–186. doi: 10.1016/s0022-5347(17)61918-9. [DOI] [PubMed] [Google Scholar]
- Blaquier J. A. Selective uptake and metabolism of androgens by rat epididymis. The prescence of a cytoplasmic receptor. Biochem Biophys Res Commun. 1971 Nov;45(4):1076–1082. doi: 10.1016/0006-291x(71)90447-5. [DOI] [PubMed] [Google Scholar]
- Clemens L. E., Kleinsmith L. J. Specific binding of the oestradiol-receptor complex to DNA. Nat New Biol. 1972 Jun 14;237(76):204–206. doi: 10.1038/newbio237204a0. [DOI] [PubMed] [Google Scholar]
- Coffey D. S., Shimazaki J., Williams-Ashman H. G. Polymerization of deoxyribonucleotides in relation to androgen-induced prostatic growth. Arch Biochem Biophys. 1968 Mar 20;124(1):184–198. doi: 10.1016/0003-9861(68)90319-6. [DOI] [PubMed] [Google Scholar]
- De Sombre E. R., Puca G. A., Jensen E. V. Purification of an estrophilic protein from calf uterus. Proc Natl Acad Sci U S A. 1969 Sep;64(1):148–154. doi: 10.1073/pnas.64.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSombre E. R., Mohla S., Jensen E. V. Estrogen-independent activation of the receptor protein of calf uterine cytosol. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1601–1608. doi: 10.1016/0006-291x(72)90897-2. [DOI] [PubMed] [Google Scholar]
- Erdos T. Properties of a uterine oestradiol receptor. Biochem Biophys Res Commun. 1968 Jul 26;32(2):338–343. doi: 10.1016/0006-291x(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Fang S., Anderson K. M., Liao S. Receptor proteins for androgens. On the role of specific proteins in selective retention of 17-beta-hydroxy-5-alpha-androstan-3-one by rat ventral prostate in vivo and in vitro. J Biol Chem. 1969 Dec 25;244(24):6584–6595. [PubMed] [Google Scholar]
- Fang S., Liao S. Androgen receptors. Steroid- and tissue-specific retention of a 17 beta-hydroxy-5 alpha-androstan-3-one-protein complex by the cell nuclei of ventral prostate. J Biol Chem. 1971 Jan 10;246(1):16–24. [PubMed] [Google Scholar]
- Fang S., Liao S. Antagonistic action of anti-androgens on the formation of a specific dihydrotestosterone-receptor protein complex in rat ventral prostate. Mol Pharmacol. 1969 Jul;5(4):428–431. [PubMed] [Google Scholar]
- HIRAOKA T., GLICK D. Studies in histochemistry. LXXI. Measurement of protein in millimicrogram amounts by quenching of dye fluorescence. Anal Biochem. 1963 Jun;5:497–504. doi: 10.1016/0003-2697(63)90069-1. [DOI] [PubMed] [Google Scholar]
- Hansson V., Tveter K. J., Attramadal A., Torgersen O. Androgenic receptors in human benign nodular prostatic hyperplasia. Acta Endocrinol (Copenh) 1971 Sep;68(1):79–88. doi: 10.1530/acta.0.0680079. [DOI] [PubMed] [Google Scholar]
- Harris G. S. Nature of oestrogen specific binding sites in the nuclei of mouse uteri. Nat New Biol. 1971 Jun 23;231(25):246–248. doi: 10.1038/newbio231246a0. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Mohla S., Gorell T., Tanaka S., DeSombre E. R. Estrophile to nucleophile in two easy steps. J Steroid Biochem. 1972 Apr;3(3):445–458. doi: 10.1016/0022-4731(72)90091-x. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Numata M., Brecher P. I., Desombre E. R. Hormone-receptor interaction as a guide to biochemical mechanism. Biochem Soc Symp. 1971;32:133–159. [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Numata M., Smith S., DeSombre E. R. Estrogen-binding substances of target tissues. Steroids. 1969 Apr;13(4):417–427. doi: 10.1016/0039-128x(69)90053-1. [DOI] [PubMed] [Google Scholar]
- Kato T., Horton R. Studies of testosterone binding globulin. J Clin Endocrinol Metab. 1968 Aug;28(8):1160–1168. doi: 10.1210/jcem-28-8-1160. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence A. M., Landau R. L. Impaired ventral prostate affinity for testosterone in hypophysectomized rats. Endocrinology. 1965 Dec;77(6):1119–1125. doi: 10.1210/endo-77-6-1119. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. A soluble androgen receptor in the cytoplasm of rat prostate. J Endocrinol. 1969 Dec;45(4):531–541. doi: 10.1677/joe.0.0450531. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. Changes in the ribonucleic acid metabolism of aging mouse tissues with particular reference to the prostate gland. Biochem J. 1968 Nov;110(1):79–86. doi: 10.1042/bj1100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring W. I., Peterken B. M. A reconstituted cell-free system for the specific transfer of steroid--receptor complexes into nuclear chromatin isolated from the rat ventral prostate gland. Biochem J. 1971 Nov;125(1):285–295. doi: 10.1042/bj1250285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring W. I. The ageing process in the mouse ventral prostate gland: a preliminary biochemical survey. Gerontologia. 1967;13(3):177–189. doi: 10.1159/000211602. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. The binding of (1,2-3H)testosterone within nuclei of the rat prostate. J Endocrinol. 1969 Jul;44(3):323–333. doi: 10.1677/joe.0.0440323. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I., Wilce P. A. Further studies on the stimulation of protein synthesis in androgen-dependent tissues by testosterone. Biochem J. 1972 Nov;130(1):189–197. doi: 10.1042/bj1300189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Mangan F. R., Mainwaring W. I. An explanation of the antiandrogenic properties of 6 -bromo-17 -hydroxy-17 -methyl-4-oxa-5 -androstane-3-one. Steroids. 1972 Sep;20(3):331–343. doi: 10.1016/0039-128x(72)90092-x. [DOI] [PubMed] [Google Scholar]
- Marver D., Goodman D., Edelman I. S. Relationships between renal cytoplasmic and nuclear aldosterone-receptors. Kidney Int. 1972 Apr;1(4):210–223. doi: 10.1038/ki.1972.31. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Atger M., Baulieu E. E. Progesterone in uterus and plasma. IV. Progesterone receptor(s) in guinea pig uterus cytosol. Steroids. 1970 Dec;16(6):741–754. doi: 10.1016/s0039-128x(70)80152-0. [DOI] [PubMed] [Google Scholar]
- Mohla S., DeSombre E. R., Jensen E. V. Tissue-specific stimulation of RNA synthesis by transformed estradiol-receptor complex. Biochem Biophys Res Commun. 1972 Jan 31;46(2):661–667. doi: 10.1016/s0006-291x(72)80191-8. [DOI] [PubMed] [Google Scholar]
- Musliner T. A., Chader G. J. A role for DNA in the formation of nuclear estradiol-receptor complex in a cell-free system. Biochem Biophys Res Commun. 1971 Nov;45(4):998–1003. doi: 10.1016/0006-291x(71)90436-0. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Poonian M. S., Schlabach A. J., Weissbach A. Covalent attachment of nucleic acids to agarose for affinity chromatography. Biochemistry. 1971 Feb 2;10(3):424–427. doi: 10.1021/bi00779a011. [DOI] [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen-binding proteins of calf uterus. Partial purification and preliminary characterization of two cytoplasmic proteins. Biochemistry. 1971 Sep 28;10(20):3769–3780. doi: 10.1021/bi00796a020. [DOI] [PubMed] [Google Scholar]
- Ritzen E. M., Nayfeh S. N., French F. S., Dobbins M. C. Demonstration of androgen-binding components in rat epididymis cytosol and comparison with binding components in prostate and other tissues. Endocrinology. 1971 Jul;89(1):143–151. doi: 10.1210/endo-89-1-143. [DOI] [PubMed] [Google Scholar]
- Rochefort H., Baulieu E. E. Récepteurs hormonaux: relations entre les "récepteurs" utérins de l'oestradiol, "8 S" cytoplasmique, et "4 S" cytoplasmique et nucléaire. C R Acad Sci Hebd Seances Acad Sci D. 1968 Aug 5;267(6):662–665. [PubMed] [Google Scholar]
- SAMUELS L. T., HARDING B. W., MANN T. Aldose reductase and ketose reductase in male accessory organs of reproduction. Distribution and relation to seminal fructose. Biochem J. 1962 Jul;84:39–45. doi: 10.1042/bj0840039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggles A. W., King R. J. The use of protamine to study [6,7-3H] oestradiol-17-beta binding in rat uterus. Biochem J. 1970 Aug;118(5):695–701. doi: 10.1042/bj1180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen H., Heyns W., De Moor P. Microheterogeneity of the testosterone binding globulin of human pregnancy serum demonstrated by isoelectric focusing. Ann Endocrinol (Paris) 1969;30(Suppl):199–203. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. In vitro conversion of estradiol-receptor protein to its nuclear form: dependence on hormone and DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2105–2109. doi: 10.1073/pnas.69.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]