Abstract
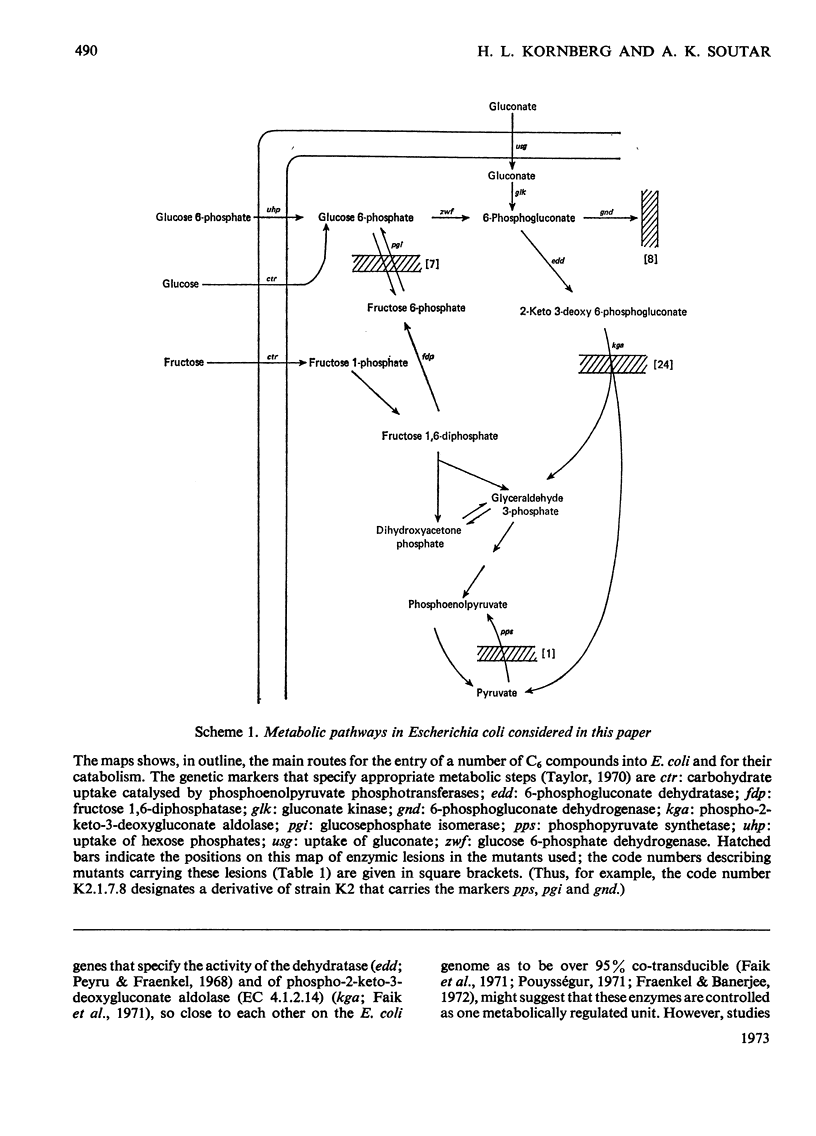
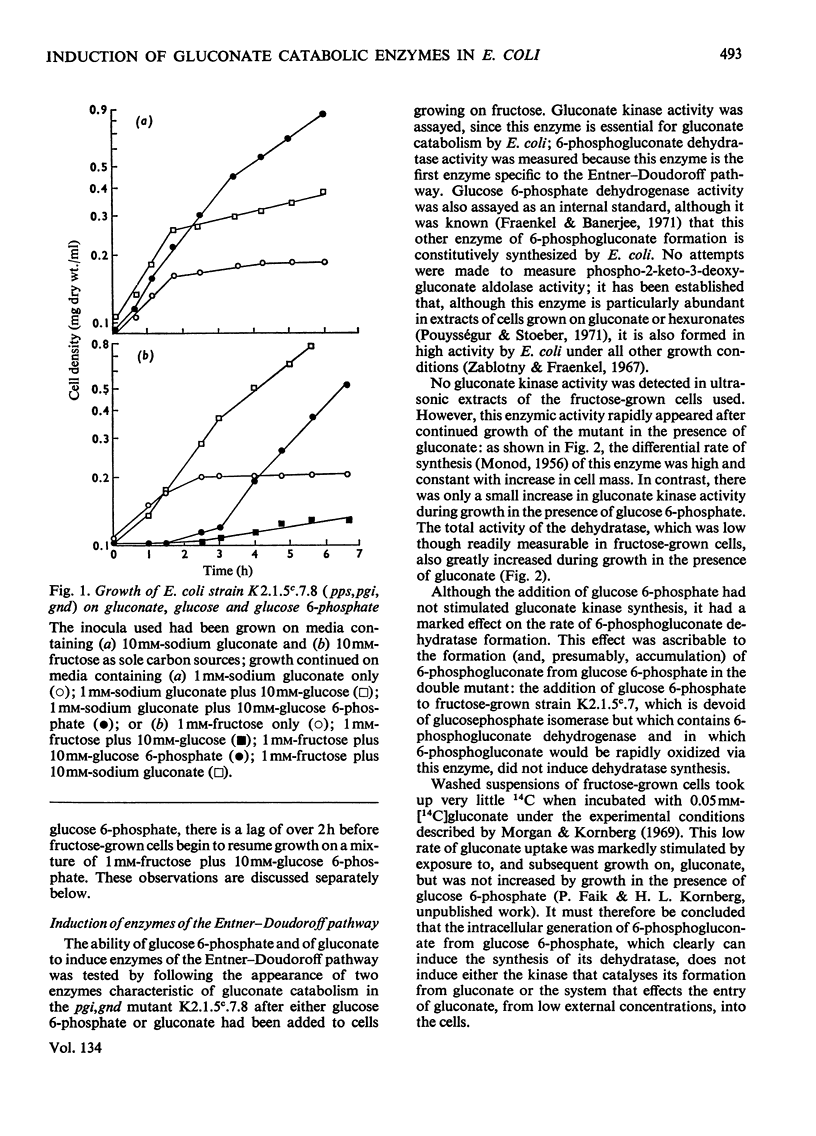
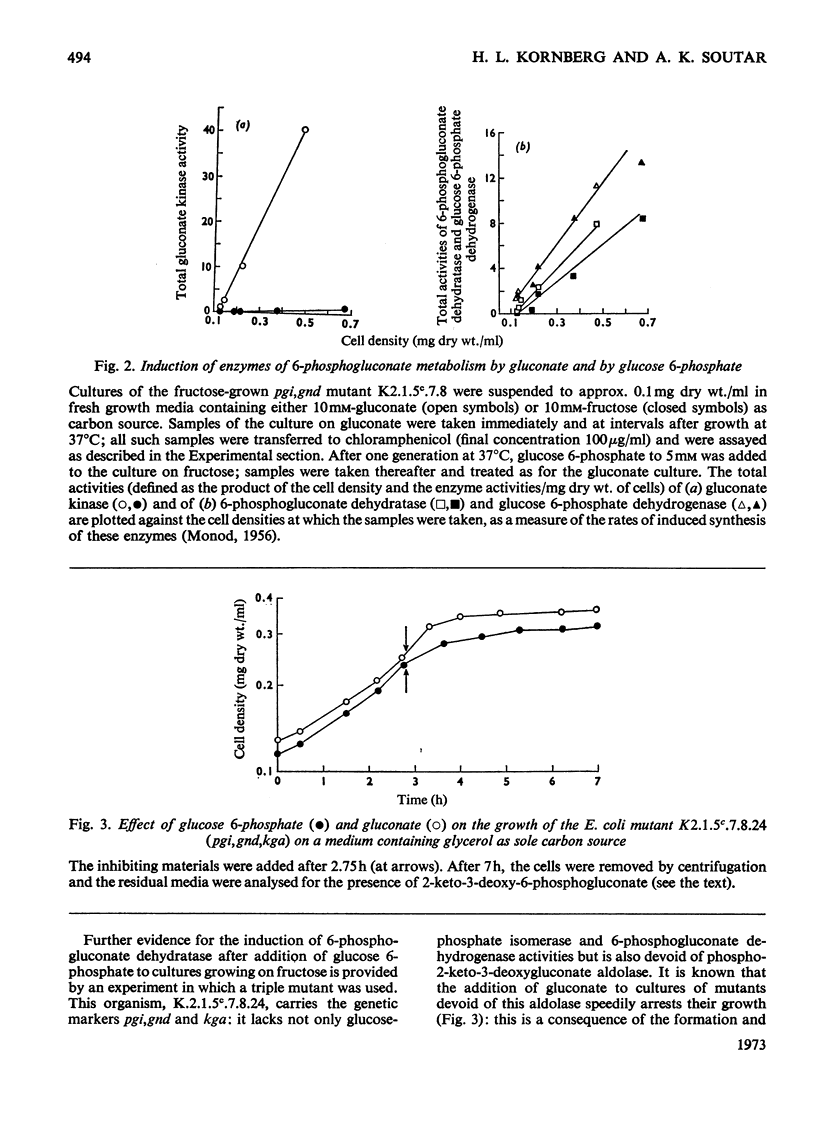
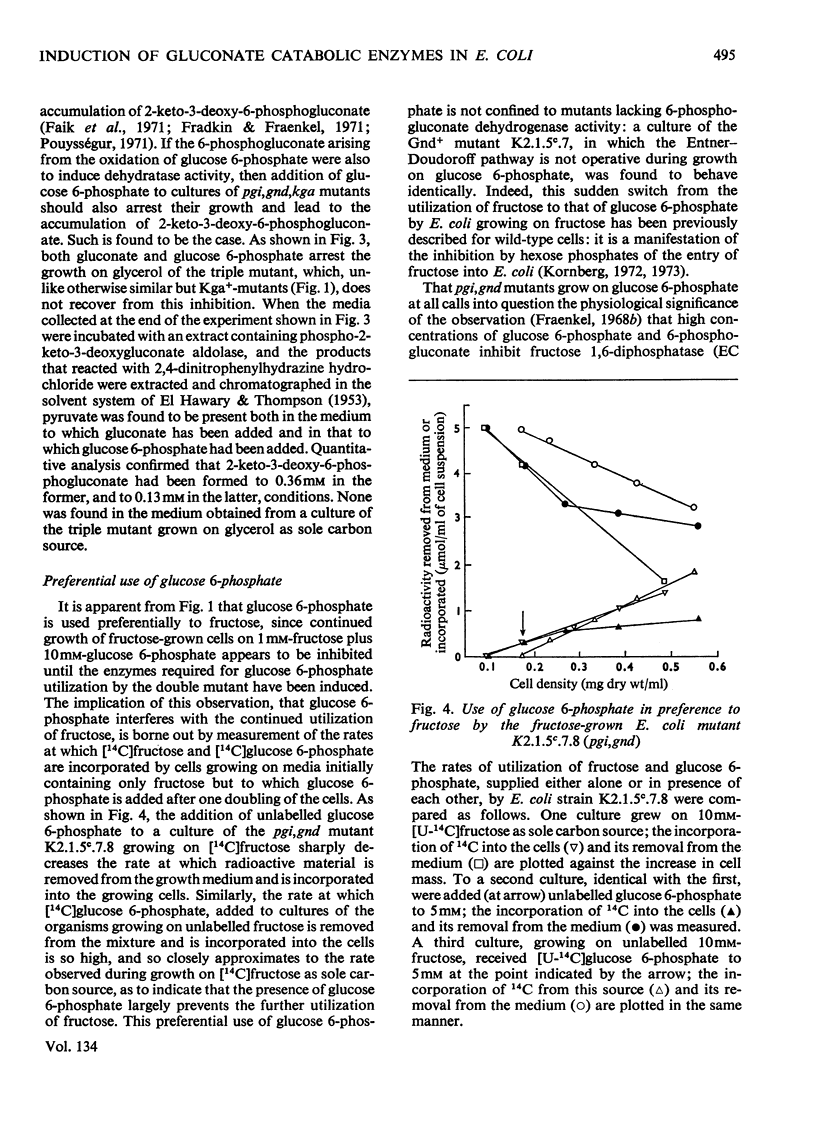
1. A mutant of Escherichia coli, devoid of phosphopyruvate synthetase, glucosephosphate isomerase and 6-phosphogluconate dehydrogenase activities, grew readily on gluconate and inducibly formed an uptake system for gluconate, gluconate kinase and 6-phosphogluconate dehydratase while doing so. 2. This mutant also grew on glucose 6-phosphate and inducibly formed 6-phosphogluconate dehydratase; however, the formation of the gluconate uptake system and gluconate kinase was not induced under these conditions. 3. The use of the Entner–Doudoroff pathway for the dissimilation of 6-phosphogluconate, derived from either gluconate or glucose 6-phosphate, by this mutant was also demonstrated by the accumulation of 2-keto-3-deoxy-6-phosphogluconate (3-deoxy-6-phospho-l-glycero-2-hexulosonate) from both these substrates in a similar mutant that also lacked phospho-2-keto-3-deoxygluconate aldolase activity. 4. Glucose 6-phosphate inhibits the continued utilization of fructose by cultures of the mutants growing on fructose, as it does in wild-type E. coli. 5. The mutants do not use glucose for growth. This is shown to be due to insufficiency of phosphopyruvate, which is required for glucose uptake.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Location of a gene specifying phosphopyruvate synthase activity on the genome of Escherichia coli, K12. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):281–292. doi: 10.1098/rspb.1967.0066. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Metabolism of carbohydrates by Pasteurella pseudotuberculosis. J Bacteriol. 1968 May;95(5):1698–1705. doi: 10.1128/jb.95.5.1698-1705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S. Gluconokinase and the oxidative path of glucose-6-phosphate utilization. J Biol Chem. 1951 Apr;189(2):617–628. [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The direct synthesis of phosphoenolpyruvate from pyruvate by Escherichia coli. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):263–280. doi: 10.1098/rspb.1967.0065. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Kornberg H. L. Properties and regulation of phosphopyruvate carboxylase activity in Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):189–205. doi: 10.1098/rspb.1966.0064. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL HAWARY M. F. S., THOMPSON R. H. S. Separation and estimation of blood keto acids by paper chromatography. Biochem J. 1953 Feb;53(3):340–347. doi: 10.1042/bj0530340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- Eisenberg R. C., Dobrogosz W. J. Gluconate metabolism in Escherichia coli. J Bacteriol. 1967 Mar;93(3):941–949. doi: 10.1128/jb.93.3.941-949.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- Faik P., Kornberg H. L., McEvoy-Bowe E. Isolation and properties of Escherichia coli mutants defective in 2-keto 3-deoxy 6-phosphogluconate aldolase activity. FEBS Lett. 1971 Dec 15;19(3):225–228. doi: 10.1016/0014-5793(71)80519-7. [DOI] [PubMed] [Google Scholar]
- Fradkin J. E., Fraenkel D. G. 2-keto-3-deoxygluconate 6-phosphate aldolase mutants of Escherichia coli. J Bacteriol. 1971 Dec;108(3):1277–1283. doi: 10.1128/jb.108.3.1277-1283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Banerjee S. A mutation increasing the amount of a constitutive enzyme in Escherichia coli, glucose 6-phosphate dehydrogenase. J Mol Biol. 1971 Feb 28;56(1):183–194. doi: 10.1016/0022-2836(71)90093-3. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G., Banerjee S. Deletion mapping of zwf, the gene for a constitutive enzyme, glucose 6-phosphate dehydrogenase in Escherichia coli. Genetics. 1972 Aug;71(4):481–489. doi: 10.1093/genetics/71.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- Kersters K., De Ley J. The occurrence of the Entner-Doudoroff pathway in bacteria. Antonie Van Leeuwenhoek. 1968;34(4):393–408. doi: 10.1007/BF02046462. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. Carbohydrate transport by micro-organisms. Proc R Soc Lond B Biol Sci. 1973 Mar 13;183(1071):105–123. doi: 10.1098/rspb.1973.0008. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem J. 1972 Aug;128(5):1339–1344. doi: 10.1042/bj1281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Role of phosphofructokinase in the utilization of glucose by Escherichia coli. Nature. 1970 Jul 4;227(5253):44–46. doi: 10.1038/227044a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T., Neidhardt F. C. Adenosine triphosphate-linked control of Pseudomonas aeruginosa glucose-6-phosphate dehydrogenase. J Bacteriol. 1967 Apr;93(4):1337–1345. doi: 10.1128/jb.93.4.1337-1345.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTLOCK R. P. Gluconate metabolism of Pasteurellapestis. J Bacteriol. 1962 Jul;84:53–59. doi: 10.1128/jb.84.1.53-59.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. J., Kornberg H. L. Regulation of sugar accumulation by Escherichia coli. FEBS Lett. 1969 Apr;3(1):53–56. doi: 10.1016/0014-5793(69)80095-5. [DOI] [PubMed] [Google Scholar]
- Peyru G., Fraenkel D. G. Genetic mapping of loci for glucose-6-phosphate dehydrogenase, gluconate-6-phosphate dehydrogenase, and gluconate-6-phosphate dehydrase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1272–1278. doi: 10.1128/jb.95.4.1272-1278.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J. M., Stoeber F. R. Etude du rameau dégradatif commun des hexuronates chez Escherichia coli K 12. Purification, propriétés et individualité de la 2-céto-3-désoxy-6-phospho-D-gluconate aldolase. Eur J Biochem. 1971 Aug 16;21(3):363–373. doi: 10.1111/j.1432-1033.1971.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Pouysségur J. M., Stoeber F. R. Rameau dégradatif commun des hexuronates chez Escherichia coli K12. Mécanisme d'induction des enzymes assurant le métabolisme du 2-céto-3-désoxy-gluconate. Eur J Biochem. 1972 Nov 7;30(3):479–494. doi: 10.1111/j.1432-1033.1972.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Quay S. C., Friedman S. B., Eisenberg R. C. Gluconate regulation of glucose catabolism in Pseudomonas fluorescens. J Bacteriol. 1972 Oct;112(1):291–298. doi: 10.1128/jb.112.1.291-298.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. A. Pathways of carbohydrate degradation in Pseudomonas fluorescens. Bacteriol Rev. 1955 Dec;19(4):222–233. doi: 10.1128/br.19.4.222-233.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotny R., Fraenkel D. G. Glucose and gluconate metabolism in a mutant of Escherichia coli lacking gluconate-6-phosphate dehydrase. J Bacteriol. 1967 May;93(5):1579–1581. doi: 10.1128/jb.93.5.1579-1581.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


