Abstract
Higher levels of physical activity are known to benefit aspects of brain health across the lifespan. However, the role of sedentary behavior (SB) is less-well understood. In this review we summarize and discuss evidence on the role of SB on brain health (e.g., cognitive performance, structural or functional brain measures, dementia risk) for different age groups, critically compare assessment approaches to capture SB, and offer insights into emerging opportunities to assess SB via digital technologies. Across the lifespan, specific characteristics of SB (particularly whether they are cognitively active or cognitively passive) potentially act as moderators influencing the associations between SB and specific brain health outcomes. We outline challenges and opportunities for future research aiming to provide more robust empirical evidence for these observations.
Keywords: prolonged sitting, cognition, dementia, children, adolescents, aging
Understanding and Influencing Sedentary Behavior to Promote Brain Health
The ways in which movement behaviors influence brain integrity and cognitive performance (brain health [see Glossary]) have been informed primarily by research on physical activity (PA) [1, 2]. It is clear from this literature that regardless of age, higher levels of moderate-to-vigorous-intensity PA (MVPA) can benefit specific aspects of brain health (e.g., cognitive performance and brain structural and functional outcomes) [2]. Conversely, the evidence concerning the role of other movement behaviors - namely sedentary behavior (SB) -- on brain health is much more limited [3, 4], although some evidence suggests that different types of SB (e.g., cognitively active SB versus cognitively passive SB) [5] might influence brain health differentially.
Given that within the 24-hour activity cycle of an individual, typically more than half of waking hours are occupied by SB while the rest of the day is spent in some combinations of light PA and a small proportion in MVPA [6, 7], the scant knowledge on the role of SB on brain health hinders the development of more specific lifestyle recommendations. The importance of a more holistic perspective considering PA and SB as unique but interrelated determinants of health outcomes [8] and underlying biology [8] is informed by the fact that PA and SB can be interdependent (for all possible interactions between PA and SB see [9]) . Accordingly, from a time-use perspective, elucidating of how SB relates to brain health is at least as important as understanding the complex relationship between PA and brain health.
Ecological models [10] and a body of evidence identify SB as a lifestyle factor that is a characteristic feature of various domains of modern daily lives, including transportation (e.g., taking the school bus), occupational activities (e.g., desk-bound office work), and leisure activities (e.g., screen-based activities such as mobile phones) [10]. Thus, it is not surprising that SB occupies a large proportion of waking hours (e.g., upwards of 60% of waking hours) across different age groups, not only in people from industrialized societies [11, 12], but also those from non-industrialized societies, even if those in non-industrialized societies spent their SB time mainly in more-active postures like squatting [13]. In industrialized societies, both self-reported and device-measured time spent in SB has increased in recent years among adolescents (≤ 19 years) [14], adults (aged 20 to 64 years) [14-16], and older adults (≥ 65 years) [14]. For instance, a large cross-sectional study in the US population observed that from 2007 to 2016 the amount of self-reported SB (e.g., operationalized by total hours spent sitting) increased from 7.0 to 8.2 hours per day among adolescents, and from 5.5 to 6.4 hours per day among adults [14]. In this context, it is worth noting that, despite their relatively high amount of leisure time (e.g., after retirement), older adults can spend an average of over 9 hours per day in SB, constituting approximately 53% to 56% of their waking hours [17] assuming an individual is awake for 16 to 17 hours per day.
The findings outlined above indicate that at any age, SB is a highly prevalent lifestyle factor due to its presence in all domains of daily living (e.g., transportation, occupation, and leisure time). This supports the proposition that, in addition to research on the influence of PA on brain health, the role of SB should also be investigated. To foster research on this issue, we (i) summarize for different age groups the evidence from observational and experimental studies (Key Figure 1) on the roles of SB and specific characteristics of SB (e.g., frequency, time, and type) on brain health, (ii) examine the potential mechanisms that might explain the associations between SB and brain health outcomes (Figure 2), (iii) discuss different assessment approaches of SB and provide an outlook on new possibilities to assess SB via digital technologies, and (iv) outline challenges and opportunities for studying the relationships between SB and brain health.
Figure 1.

Sedentary behavior and brain health outcomes: Evidence from randomized controlled trials is presented across the lifespan and three different levels of analysis. The life span in this study is divided into relatively broad age ranges [1] including preschool period (ages 3 to 6 years); middle childhood (ages 6 to 12 years); adolescence (ages 12 to 20 years); young adulthood (ages 20 to 40 years); middle adulthood (ages 40 to 65 years); and late adulthood (ages 65 years to death). The question mark within the blank area indicates an existing gap in evidence while outcome measures in each column reflect emerging pattern on associations between sedentary behavior and brain health.
Figure 2.

The potential mechanisms that might explain the association between sedentary behavior and brain health: (i) changes on molecular and cellular levels (e.g., brain-derived neurotrophic factor) [2, 3], (ii) functional and structural brain changes (i.e. grey matter volume)[4-8], and (iii) socioemotional changes (e.g., stress, sleep)[9, 10]. Changes of the above-mentioned levels of analysis have all been shown to mediate the effects of physical activity on cognitive performance [11], and thus may be good candidates to include in studies seeking to understand the role of SB in brain health. Notably, despite age and health status, cognitively active SB may benefit specific aspects of brain health [12, 13] , whereas cognitively passive SB are more consistently linked to poorer cognitive performance [12, 13]. BDNF, brain-derived neurotrophic factor; IGF-1, insulin-like growth factor.
Associations of Sedentary Behavior with Brain Health in Children and Adolescents
Among children and adolescents, emerging evidence on the associations of SB with brain health stems from observational data using cross-sectional and longitudinal designs, with sample sizes ranging from 19 to 4304 participants [18-35]. Previous studies have focused on cognitive domains including attention [18, 19, 23], visuospatial cognition [21, 22], visual memory [23], executive function [20, 23-25], and intelligence [26, 28-30, 32, 33, 35]. Collectively, the evidence on the role of SB on behavioral outcomes is mixed among children and adolescents. For example, duration (or frequency) of watching television (TV) was negatively linked to cognitive performance (e.g., attention and learning abilities) in both cross-sectional and longitudinal studies [18, 19, 21], but such associations were not observed in other studies with cross-sectional [27] or longitudinal designs [34]. Additionally, Swing and colleagues found a positive relationship between hours of video game playing and attention problems [21], while its relationships with other cognitive measures (e.g., verbal, numeric and reasoning abilities) were not significant [27]. Closer examination of these studies revealed that key covariates (e.g., sleep quality and PA level) [36] were seldom collected, which may explain the mixed findings.
In contrast to studies using behavioral measures of brain health, a small number of studies have examined associations of SB with brain-related biological markers. For instance, device-measured sedentary time was not linked to P3 amplitude and latency (recorded via electroencephalography [EEG]) during inhibitory control task among children with overweight/obesity [25]. Functional magnetic resonance imaging study indicated [26] that hours of reading (cognitively-active SB) was positively linked to greater functional connectivity between the visual word form area and language-related (e.g., left Brodmann 40, 42, 43 and 22), visual (e.g., bilateral Brodmann 19) and cognitive control (e.g., left Brodmann 7 and 44) regions; it also showed that time spent in screen-based media activities (e.g., smartphones, tablets, TV) was linked to lower connectivity between the visual word form area and regions related to language (e.g., bilateral Brodmann 39 and 20) and cognitive control (e.g., right Brodmann 24 and 13) [26]. These observations suggest that different types of SB (i.e., reading versus screen-based media exposure) differentially influence neurocognitive measures of brain health in children. This assumption is supported by studies investigating the influence of different types of SB on structural brain measures assessed via structural magnetic resonance imaging. For instance, in the ActiveBrains project the baseline data was used to investigate associations of SB (device-measured and self-reported sitting time and type) with brain structure [30-32] and they observed that sedentary time and SB type (e.g., watching TV) were negatively linked to gray matter and subcortical brain volumes (e.g., right hippocampus, left thalamus and right nucleus accumbens) among children with overweight/obesity [31, 37, 38], while gray matter in the cerebellum was positively linked to crystallized intelligence [38]. Such findings are partially consistent with the observations of another study conducted among normal-weight children [29], which indicated that frequency of internet use was negatively and longitudinally linked to gray matter volume of a widespread brain network (e.g., bilateral perisylvian area, cerebellum, and subcortical regions [e.g., hippocampus, amygdala or basal ganglia]).
Collectively, the findings described above suggest that screen-based activities (acknowledged as a cognitively passive SB), appear to be detrimentally linked to behavioral and neurological measures of brain health. However, before making a definitive conclusion, dose-response and type of SB should be considered since not all screen time, including potentially some SB’s that may be considered to be cognitively passive, may not be necessarily detrimental for brain health. For instance, moderate duration of video gaming appeared to be beneficial for cognitive performance [39], whereas long duration of gaming may be bad for academic achievement (Box 1) [40].
Box 1. Importance of educational activities on academic achievement.
Cognitive abilities are deemed to be the basis of the development of academic performance among children and adolescents [104]. However, it remains relatively unclear whether screen media use (one of the most prevalent leisure-time SBs among children and adolescents) is linked to academic performance. A meta-analysis synthesized the results from 58 cross-sectional studies and reported that TV watching was negatively linked to composite academic performance scores, language, and mathematics while video game playing was negatively linked to composite academic achievement alone. Further, a subgroup analysis indicated that watching TV was negatively linked to language skills in children, whereas both TV and video game playing were negatively linked to composite academic achievement in adolescents [40]. In a cross-sectional analysis, Sánchez-Oliva and colleagues [105] observed that children and adolescents with a time-use SB profile focused on educational activities (e.g., doing homework with/without a computer and reading for fun) demonstrated better academic performance as compared to other time-use profiles (screen time [e.g., TV viewing, video game playing and internet surfing], social time [e.g., talking or chatting on the telephone], or relaxing time [e.g., sitting while doing nothing, a jigsaw and crossword puzzles, chess or checkers]). Further, a longitudinal analysis indicated that boys who changed from a screen- to an educative profile reported better academic performance as compared to boys who changed from an educative- to a social- or screen-SB profile. Taken together, SB type (reading/stimulating activities vs. TV/screen time) seems to differentially relate to academic performance [105]. Notably, to deepen understanding regarding the differential effects of SB type on cognitive aspects of brain health, including academic achievement among children and adolescents, quantitatively classifying these two SB types and dose-response relationship of each SB type (e.g., low duration of cognitive passive SB may not be detrimentally linked to brain health) should be prioritized in future studies.
Associations of Sedentary Behavior with Brain Health in Younger Adults
Two cross-sectional studies provide evidence that time spent in sitting bouts lasting at least 20 consecutive minutes and sitting of > 3 hours were associated with poorer inhibitory control [41] and lower working memory performance [42] among young adults, when the influence of other movement behaviors was statistically controlled (e.g., different intensity levels of PA such as low-intensity, moderate-intensity, and vigorous-intensity PA, and/or daily total sedentary time). Furthermore, in a large-scale study (N =3247), adults with an average age of 25 years who were categorized as watching higher amounts of TV (> 3 hours/d) had poorer processing speed and executive function compared with those watching lower amounts of TV [43], even after adjusting for important covariates including BMI, smoking, blood pressure, and apolipoprotein E4 phenotype. Further, younger adults who spent a large number of their waking hours in watching TV and less time in PA, had nearly twice the odds of poor performance on executive function and processing speed as compared to those with the opposite behavioral pattern (i.e., low TV consumption and high PA engagement) [43].
Researchers have also begun to examine possible neurobiological processes that drives the relationships between SB and cognitive performance. For instance, in a cross-sectional study a hip-worn accelerometer was used to estimate SB to examine links with executive function-related EEG markers among adults with overweight/obesity [41]. Findings indicated no significant associations of daily sedentary time and time spent in sitting ≥20 min with P3 latency related to inhibitory control and cognitive flexibility. Another cross-sectional study in younger adults investigated potential associations of accelerometer-based SB with decision-making competence using graph theoretical network analysis [44]. Findings indicated concurrent and opposing associations with susceptibility to framing (cognitive biases) in a sample of young, aerobically fit adults. Further, sedentary time was related to decreased functional connectivity of the dorsal attention network, which mediated a positive association between sedentary time and susceptibility to framing [44]. This mediated effect suggested that sedentary time may be associated with lower readiness for top-down control, which in turn may contribute to a greater susceptibility to framing [44]. However, sedentary time was also positively related to lower susceptibility to framing after accounting for the mediated effect. Thus, this study underscores the need to assess moderators of the sedentary time-cognition (e.g., decision-making, planning, reasoning) such as SB type.
The number of observational studies on this research topic is rapidly growing, but there is still insufficient evidence to draw robust conclusions regarding the role of SB in brain health of younger adults, especially across finer-graded developmental periods (e.g., emerging adulthood) [45]. Additionally, in these age groups future prospective studies and controlled intervention trials including healthy and clinical populations are needed.
Association of Sedentary Behavior with Brain Health in Middle-aged and Older Adults
The current evidence concerning the associations of SB with cognitive performance in middle-aged adults (40-65 years) and older adults (> 65 years) is mixed [3, 46-48]. Some systematic reviews and meta-analyses, which are largely based on observational studies, have reported that lower levels of SB are linked to better cognitive performance (e.g., global cognitive performance) [47, 49], while others did not find such a relationship [48] or concluded that the available evidence is too premature for robust conclusions [3, 46, 50]. In this context, results of a clinical study indicated that the association between SB and cognitive performance might vary as function of cognitive status because higher levels of PA and lower levels of SB were linked to better global cognitive performance in older adults without mild cognitive impairment (MCI), but not in older adults with probable MCI, although the latter spent more time in SB [51]. Likewise, the associations between SB and the risk of neurodegenerative diseases (e.g., Alzheimer’s disease) are also somewhat mixed, although a recent meta-analysis indicated that more time spent in SB is associated with a higher incidence of dementia [52]. For example, while some work suggests that SB was not linked with the risk of dementia in older women [53], another recent study showed that average daily SB had a non-linear association with incident dementia [17]. Further, among a sample of ~50,000 UK participants, spending more than 10 hours a day in SB was associated with higher risk for dementia, even after accounting for MVPA [17]. This mixed evidence is at least partly attributable to methodological limitations of previous work, including the utilization of questionable assessment methods of SB (e.g., questionnaires with poor or unknown validity) [49], and/or non-consideration of important moderators such as different types of SB (Box 2) and MVPA in the analyses [3, 46]. Such methodological limitations may also explain the mixed findings that has been observed regarding biological measures of brain health and will be discussed in the following in more detail.
Box 2. Type of sedentary behavior differentially linked to brain health in middle-aged and older adults.
Previous research suggests that SB type is an important factor that can modify the SB-cognition relationship. For instance, some observational studies reported in older adults that the time spent in cognitively passive SB (e.g., TV viewing) was associated with worse cognitive performance [50, 106] and a higher risk of dementia [107], whereas the time spent in cognitively active SB (e.g., computer use) was associated with better cognitive performance [108, 109] and a lower risk of dementia [107]. The idea that cognitively active SB, or at least, specific forms of cognitively active SB, can benefit cognitive performance is partially supported by meta-analyses showing that in healthy older adults computerized cognitive training, which is typically conducted in a sitting position, improves cognitive performance in specific domains [110]. Nevertheless, it is acknowledged that the evidence on the effectiveness of cognitive training (e.g., on general cognition) is not universal and controversially debated [111]. Notably, the relationships between cognitively passive or active SB have been shown to persist even after accounting for important covariates (e.g., level of regular PA operationalized by MET hours per week [85, 106]), which supports the likelihood that cognitively passive SB may be an independent risk factor for the decline in cognitive performance and the risk to develop dementia. However, based on a limited pool of studies that have investigated the influence of different characteristics of SB on cognitive performance in middle-aged and older adults [3, 46, 50], future research that (i) specifically focuses on the effects of different characteristics of SB and (ii) considers the joint effect of time spent in different intensity levels of PA is necessary. In this context, it is worth noting that in older adults the evidence for the positive effects of MVPA on parameters of brain health (e.g., cognitive performance) is robust (for review see [1, 112]) whereas the body of evidence documenting the positive role of standing [113] and light-intensity PA [114] is emerging. The assumption that examining the joint effects of PA and SB is of importance to derive more nuanced recommendations is supported by the findings of a large observational study in the aging UK population (n = 484,169) reporting that the daily replacement of 30 minutes of leisure-time SB with an equal amount of PA can decrease the risk of all-cause dementia, Alzheimer Disease, and vascular dementia by 6%, 5%, and 7%, respectively [115].
Findings from observational studies
The current evidence on the influence of SB on biological measures of brain health is scarce and lacks consensus. For instance, on the one hand, cross-sectional studies observed that in older adults, a higher amount of SB was associated with lower serum levels of brain-derived neurotrophic factor (BDNF) [54], lower cortical thickness of the medial temporal lobe [55], lower cerebral blood flow in lateral and medial frontal regions [56], and lower white matter volume [57] - while some effects of SB persisted even after controlling for the level of MVPA (e.g., on cerebral blood flow [56] or white matter volume [57]. On the other hand, other cross-sectional studies observed that the time spent in SB was not correlated with hippocampal volume [54] and cortical thickness (e.g., temporal pole and superior frontal gyrus) [58] when accounting for MVPA [58]. Conversely, a cross-sectional study reported lower hippocampal volumes and increased white matter in older adults spending more than 8 hours per day in SB (versus less than 8 hours), but no significant differences in prefrontal or global cerebral hemodynamics [59]. Notably, although there is evidence that in older adults changes in BDNF [60, 61] and hippocampal volume [62-64] mediate the relationship between PA and cognition, none of the above-mentioned studies elucidate whether the observed SB-related differences in biological measures of brain health have mediated a potential relationship between SB and cognitive performance. Thus, from the evidence of the above-presented observational studies it remains open whether the effects of SB are agnostic to that of PA or may constitute an independent risk factor for brain health.
Findings from interventional studies
Complementing the observational study evidence, randomized crossover trials have begun to investigate the combined effects of PA and SB on brain health. For instance, one study in older overweight adults reported that acute physical exercise has an acute positive effects on executive functioning depending upon whether the acute bout of physical exercise was followed by prolonged sitting [65]. Such cognitive improvement may be attributable to exercise-induced increase on concentrations of BDNF [65]. In the same study, prolonged sitting impaired cerebral blood flow, but the acute exercise mitigated this effect [66]. Interestingly, when acute physical exercise was followed by a prolonged bout of sitting, the time spent sitting did not attenuate the benefits of acute physical exercise on cerebral blood flow, but it did attenuate the benefits of acute physical exercise on blood pressure [67] – an important longer-term risk factor for cognitive decline.
The current state of research findings does extend the evidence from acute intervention studies to the longer term. For instance, an 6-month intervention study in middle-aged and older adults with osteoarthritis, found no effect of increasing MVPA and reducing SB on episodic and working memory [68]. Given that osteoarthritis is a risk factor for cognitive decline any positive effect of increased MVPA and reduced SB may be offset by an osteoarthritis-related decline in cognitive performance [69]. Additionally, an 8-week E-health intervention to reduce SB in the workplace found also little to no improvement in cerebrovascular function, productivity, or focus [70] which is partially consistent with another 16-week intervention showing that reduced sitting resulted in increased resting cerebral blood flow, but no change in cerebrovascular function [71].
As discussed earlier, there are multiple ways to assess the SB reduction effects on brain health. Some studies have observed improvements of some indicators of brain health, but others have not – leaving questions as to the longer-term effects. That said, longer-term interventions targeting SB seem to more consistently improve cardiometabolic risk factors for cognitive decline – such as glucose/insulin regulation and blood pressure [72]. This suggests that future studies should include an expanded set of outcomes when trying to capture the implications of SB for brain health over the longer term including, but not limited to (i) changes in molecular and cellular levels (e.g., BDNF), (ii) functional and structural brain changes (i.e., functional connectivity, grey matter volume), and (iii) socioemotional changes (e.g., stress, sleep). Changes in these factors have all been shown to mediate the effects of PA on cognitive performance [2], and thus may be good candidates to include in studies seeking to understand the processes through which SB may affect brain health (Figure 2). Additionally, future research should especially consider the moderating influence of the type of SB given the preliminary evidence from a small-scaled study (n = 33) suggesting that cognitively active SB (e.g., 6-month video game training with 5 days/week x 30-min session/day) could lead to an increase in the brain volume [39] which otherwise is susceptible to age-related atrophy (e.g., hippocampal volume) [73]. Given preliminary finding engaging in specific types of SB (i.e., cognitively active SB) that may have some potential to contribute to a preservation of brain health in middle-aged and older adults, future research in this direction is needed to substantiate this initial evidence.
Moreover, in addition to the view of brain-related measures as outcomes, using brain imaging data as a predictor of engaging in SB is a promising avenue for future investigations (Box 3) because gaining knowledge in this direction might help to identify individuals who needs additional support for lifestyle changes (e.g., PA engagement) being important to preserve or increase brain health in middle-aged and older adults.
Box 3. Brain parameters as predictors of sedentary behavior.
There is evidence from PA studies in older adults that specific brain parameters (e.g., higher functional connectivity in default, frontoparietal, and attentional networks, gray matter volumes, and white matter volumes of specific brain areas including, but not limited to frontal brain regions and corpus callosum) can predict adherence to PA [116], (i.e., there are brain attributes that can be considered as potential predictor variables [117]). With regard to the role of brain attributes as predictors of SB, an observational, longitudinal study in a cohort of Icelandic older adults (n = 352) reported (i) that higher amount of time spent in SB is associated with lower volume of white matter (i.e., at follow-up at 5 years and 5-year change) even after adjusting for important covariates including the baseline values of self-reported hours spent in moderate-intensity and vigorous-intensity PA, and (ii) that a higher 5-year atrophy of white matter volume predicted higher levels of SB [57]. These findings provide preliminary evidence for a bidirectional relationship between SB and brain health which is supported by findings on behavioral level [118]. Broadening our understanding of the role of brain parameters as predictors can offer a unique opportunity to identify middle-aged and older adults who are more predisposed to SB and who, in turn, may need additional support to reduce time spent in SB [117] .
Assessment of Sedentary Behavior
In parallel with the multiple ways in which brain health can be measured, there are also multiple ways to measure SB. It is worth noting that these different measurement approaches can have pros and cons (Figure 3) and because of this, can yield different insights into relationships with brain health.
Figure 3.
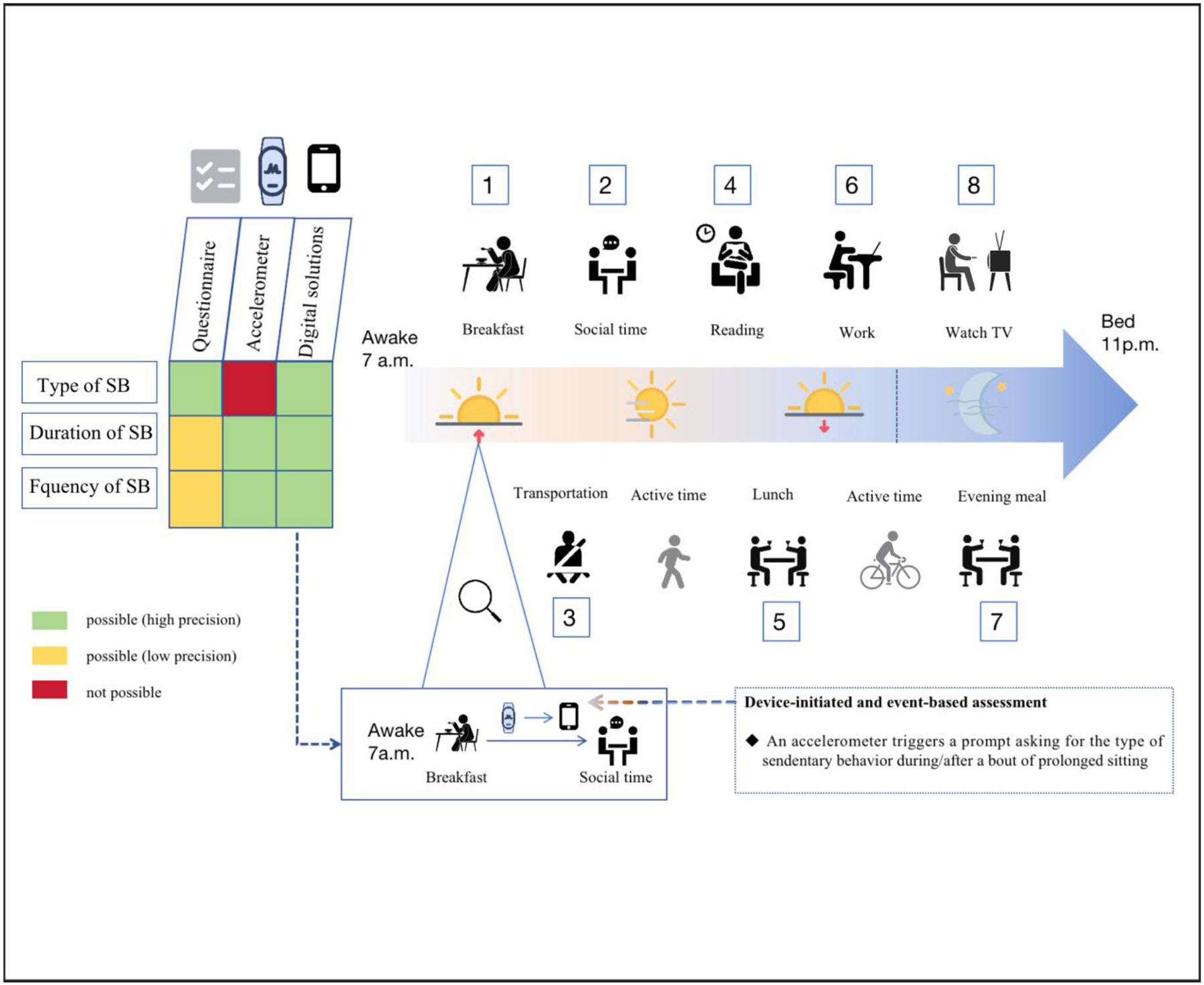
Strengths and limitations of different measurement assessment of sedentary behavior. For type of sedentary behavior, duration of sedentary behavior, and frequency of sedentary behavior, Green, Yellow, and Red represent their respective levels (possibly high, possibly low, and not possible) of precision across measurement approaches including self-reported questionnaire, accelerometer, and digital solutions. A large proportion of waking hours are occupied by sedentary behaviors within the (approximate) a typical 24-hour activity cycle of an adult. The numbers are arranged in ascending order (1 = breakfast, 2 = social time, 3 = transportation such as taking the school bus, 4 = reading, 5 = lunch, 6 = work, 7 = evening meal, 8 = TV watching. The above-mentioned activities can be measured via Questionnaire, Accelerometer, and/or Digital solution.
Specifically, SB can be assessed using self-reports (e.g., via questionnaires) or device-based methods (e.g., via accelerometry) [74]. While self-reports allow for a cost-efficient assessment of the time and context of SB (e.g., questionnaires to assess time spent in cognitively active or cognitively passive SB [75]), this assessment method is prone to several sources of bias (e.g., recall bias or social desirability bias that can lead to the underestimation of sedentary time) [76]. The latter is perhaps related to the fact that sitting time itself is less accessible in cognitive representations because it is perceived as an incidental component of activities that are performed during sitting (e.g., computer use, reading) and is, in turn, not reliably encoded [77]. Thus, it is recommended that assessments using self-reports should focus on the quantification of the time being spent in seated activities, which can also be used to indirectly determine the total sitting time, rather than attempting to measure total sitting time via direct recall [77]. Furthermore, another disadvantage of self-reports arises from the issue that several studies investigating the role of SB on brain health utilized questionnaires that have not been psychometrically evaluated (e.g., unknown validity) [49, 50] . Given that validity and reliability are cornerstones of robust and replicable findings [78], such practices not only call the current findings into question, but also are a call for future efforts to implement more rigorous assessments [49].
Conversely, device-based methods ensure a relatively more precise assessment of the (total) time spent in SB, as well as allowing indicators of SB patterns to be derived (e.g., mean duration of SB bouts). However, they do not provide contextual information on SB [74], limiting their ability to understand how cognitively passive and cognitively active SB influences brain health. Furthermore, there is not yet a fully established consensus on the application of device-based measurement tools (e.g., placement and sampling frequency of the device) and the data processing procedures to obtain specific measures of PA and SB (e.g., minimal length of the epochs, filter, cut-off points, non-wear-time definition) [79], which is a challenge for comparison across studies. In this context, there is some evidence that algorithms based on machine learning might allow for a more accurate assessment of SB as compared to traditionally used fixed cut-off points [80], although future research in this direction is required before such approaches can unreservedly be recommended and applied [79].
Even if the combination of self-reports and device-based assessment methods will not circumvent all issues challenging the accurate assessment of different characteristics of SB, utilizing the strengths of both methods within a single assessment might be the most promising approach to yield a more nuanced and robust understanding regarding the role of different characteristics of SB (e.g., type, frequency, and duration) on specific outcomes of brain health [74]. The need to apply more accurate SB assessment methods is supported by the findings of a systematic review reporting that the associations between SB and cognitive performance are weak and vary based on both the measurement method that is used to quantify the total sedentary time (e.g., self-reports vs. device-based assessment) and the cognitive domains being assessed [46].
Future research to improve the assessment of SB may benefit from the application of digital technologies (e.g., ecological momentary assessments), particularly in terms of generating findings in ecologically valid settings [81]. In this regard, the application of digital technologies (e.g. using smartphones) might also allow researchers to tackle the issue that self-reports and device-based measures are typically assessed over a limited time frame (e.g., seven days), thereby permitting only a limited snapshot of PA and SB [50, 82]. Furthermore, the utilization of digital technologies such as miniaturized and wearable cameras can also enable an objective assessment of contextual information (e.g., identifying dietary patterns such as snacking during television viewing) and identification of the SB type – promoting their application is ethically feasible [74]. Such evidence may yield a better understanding of the context and role of different characteristics of SB on measures of brain health. However, the application of innovative assessment methods (e.g., ambulatory assessment using miniaturized and wearable sensors) is not without limitations (e.g., lacking standardization, ethical considerations) [83].
Challenges and Potential Solutions in Studying Relationships of Sedentary Behavior with Brain Health
SB-induced changes in brain health across the lifespan remain largely understudied. Future studies should be conducted to determine the nature of the association: (1) the need for larger study samples, longitudinal designs, and longer follow-up periods in order to better understand the time-course by which SB impacts brain health, given that most of the current evidence comes from smaller samples and cross-sectional studies; (2) emphasizing the importance of including both device-measured SB and self-report in study protocols in order to capture the behavior and contexts; (3) standardized neuropsychological assessments using tools with good evidence of validity and reliability.
To gain a better understanding of the complex relationships of SB with the many dimensions of brain health, experimental models should be considered in future research, including animal studies on basic mechanism. A seminal review [8] indicated that hindlimb unloading model and wheel lock methodologies have been most-extensively used in rats, while SB-induced physiological changes in humans have been inferred from multiple experimental models, including bed rest, limb immobilization, reduced daily step count, interrupting prolonged sitting [8]. Notably, interrupting sitting time in laboratory-based and free-living settings is a relatively innovative and ethically acceptable approach that is now being increasingly used in SB research. However, such studies often do not control for the nature of the cognitive activity being performed in a seated position during SB: Participants in the control condition are usually allowed to perform homework or reading [84] rather than simply sitting for 2 hours, which is not an appropriate comparison condition. Since cognitively active SB (i.e., reading) can be linked to cognitive and brain benefits [85], the inclusion of a control group that engages in cognitively passive activities (e.g., TV viewing) is recommended for future studies.
Further, as can be the case in PA interventions, random assignment in such SB trials may not be easily implemented or manipulated. To this end, statistical mediation should be considered to identify the potential mechanism or to examine the putative pathways that may underlie observed associations between an independent variable and a dependent variable via an intermediary variable. In such mediation models, researchers can gain helpful insights into whether SB – for example, total sitting time or frequency of television viewing may be indirectly linked to long-term memory or executive function via reduced hippocampal volume or decreased functional connectivity. Also, Mendelian randomization is a statistical method using genetic variation to examine potential causal effects of modifiable exposures on different outcomes of interest [86]. Such statistical methods have been increasingly used in observational epidemiological studies, including investigations of relationships between PA and psychiatric disorders (e.g., Alzheimer’s disease) [87, 88]. Future observational studies might consider Mendelian randomization in SB-cognition research when genetic variants related to modified factors such as SB time and type exist.
In terms of outcomes, attention, executive function, and intelligence as specific behavioral manifestations have primarily been measured in previous studies, especially in children and adolescents. Other types of cognitive functioning including language, somatosensory, social function, and reasoning and problem-solving should be included in future studies. Additionally, several single-group pre-test/post-test studies have included central (e.g., cerebral blood flow, corticospinal excitability) [71, 89, 90] and peripheral measures (e.g., catecholamines) [91] among adults who were exposed to prolonged, uninterrupted sitting. Such biological markers should be included in future well-designed studies in combination with imaging techniques such as fMRI and EEG and/or eye-based measures for more definitive conclusions on SB-induced effects on cognition [92, 93].
While examination of the differential associations of SB type with aspects of brain health is attracting increasing attention, it is equally important to examine the various other characteristics of SB (e.g., frequency and time). Lastly, SB is often accompanied by other unhealthy behaviors (e.g., snacking or drinking) [94] which, in turn, might drive some of the observed effects and thus should be considered as confounders in future studies.
Concluding Remarks and Research Priorities
Our current understanding of how waking movement behaviors – namely PA and SB – influence cognitive and brain health is predominately based on PA research findings, whereas our knowledge concerning the influence of SB on brain health (e.g., cognitive performance, dementia risk, and biological measures) is more limited and mostly of studies focus on middle-aged and older adults. However, our review summarizing the current state of the literature on the associations between SB and brain health suggests that, in addition to other sources of heterogeneity, does-response (e.g., type, duration, frequency, timing) and the type of SB may constitute important moderators to be further investigated. Regardless of age and health status, cognitively active SB may benefit specific aspects of brain health, whereas cognitively passive SB are more consistently associated with poorer cognitive performance. Lastly, PA break seems to be effective in mitigating adverse effects of SB on brain health, which should be further investigated in well-designed studies before making a firm conclusion.
In proposing future research opportunities (see Outstanding Questions), we suggest that future studies should seek to better understand and substantiate the evidence of how different characteristics of SB influence brain health across the lifespan by (i) utilizing innovative measurement and analytic approaches to capture SB within a 24-hour activity cycle in ecological settings (e.g., via ambulatory assessment using miniaturized and wearable sensors); and, (ii) considering SB-related neurobiological mechanisms at different levels of analysis that are relevant for brain health in general and cognitive performance in particular (e.g., functional and structural brain changes).
Outstanding questions.
Five key research questions should be priorities for future investigation:
Does SB, as compared to PA, constitute an independent risk factor that negatively influences cognitive performance? Do the potential effects vary as a function of age and/or health status?
Do different characteristics of SB differentially influence performance in specific cognitive domains? Do the potential effects vary as a function of age and/or health status?
Which neurobiological mechanisms may drive the effects of different characteristics of SB on cognitive performance? Do these neurobiological mechanisms vary as a function of age and/or health status?
Does a bidirectional relationship between SB and specific parameters of brain health exist? If so, does such a bidirectional relationship change as a function of age and/or health status?
Can specific domains of baseline cognitive performance (e.g., executive functions) or functional or structural brain parameters predict future levels of SB?
Highlights.
The evidence regarding the influence of sedentary behavior (SB) on brain health – namely cognitive performance, dementia risk, and biological markers - is mixed, and most of the studies focus on middle-aged and older adults.
The type of SB (e.g., cognitively active SB vs cognitively passive SB) appears to be differentially linked to brain health (e.g., high-order cognitive function, academic achievement, and dementia risk), but further studies considering the dose-response relationships (e.g., type, duration, frequency, timing) are needed to verify such a relationship further.
Evidence is needed on how interrupting prolonged sitting with physical activity breaks mitigates potential adverse impacts of SB on specific aspects of brain health.
Future studies should utilize innovative approaches to capture SB within the 24-hour activity cycle in ecologically valid settings (e.g., via ambulatory assessment), assess the characteristics (e.g., type, duration, frequency, timing) of SB, and consider different levels of analysis (e.g., molecular/cellular changes, functional and structural brain changes, and socio-emotional changes) to reach robust conclusions on relationships and mediators of SB and brain health.
Acknowledgement
This study was supported by The Shenzhen Educational Research Funding (zdzb2014), The Shenzhen Science and Technology Innovation Commission (202307313000096), The Social Science Foundation from the China’s Ministry of Education (23YJA880093), The Post-Doctoral Fellowship (2022M711174), and The National Center for Mental Health (Z014).
Glossary
- Brain health
This refers to the optimal development and preservation of brain integrity which encompasses (i) structural (e.g., grey matter volume) and functional (e.g., functional connectivity) brain parameters, (ii) parameters of mental and cognitive performance, and (iii) the absence of neurological disorders [95].
- Graph theoretical network
refers to a type of network analysis to assess the efficiency of information processing within and across intrinsic connectivity brain networks [44].
- Framing effect
is a cognitive bias in which a person reacts to a choice or concept according to how it is presented or construed [96].
- Characteristics of SB
These include type (i.e., cognitively active, or cognitively passive), frequency (i.e., the number of bouts of SB over a given time frame, typical bout lengths are ≥ 30 minutes, ≥ 60 minutes, or ≥ 120 minutes), and time (i.e., the total duration of time spent in SB or accumulated time spent in bouts of uninterrupted, prolonged sitting). For example: increasing sedentary time results in mal-adaptations in physiological systems [5, 8].
- Emerging adulthood
refers to the developmental phase that spans from late adolescence to adulthood (age range between 18 and 29 years old) [45].
- Cognitively active SB
This refers to SB that requires a relatively high level of cognitive engagement (e.g., reading, playing video games, sitting and talking to others, driving a motor vehicle) [5].
- Cognitively passive SB
This refers to SBs that require a relatively low level of cognitive engagement (e.g., watching television, sitting as a passenger while commuting)[5].
- Electroencephalography
is a mobile neuroimaging method that is based on recording spontaneous and event-related electrical activity of the brain [97].
- P3
is a positive-going component of a stimulus derived event-related potential (ERP) elicited in the process of decision making and it consists of two parameters: (a) amplitude is recognized as a marker of inhibition and proportional to the amount of attentional resources allocated during stimulus engagement; (b) latency is thought to be proportional to stimulus evaluation and information processing speed [98].
- Magnetic resonance imaging
is a noninvasive neuroimaging technique that can be used to measure and map brain activity [99] – also known as functional magentic resonance imaging or to assess structural brain changes – also known as structural magnetic resonance [100].
- Movement behaviors
These include all behaviors that occur in a 24-hour cycle of a day (i.e., also referred to as time-use behaviors). Typically, the term includes waking behaviors such as sedentary behavior and physical activity, and non-waking behaviors such as sleep[101].
- Physical activity
This is defined as all muscle-induced bodily movements (e.g., in occupational or leisure time) leading to an increase in the energy expenditure above ∼1.0/1.5 MET (metabolic equivalent of the task; 1 MET= 1 kcal (4.184 kJ) • kg− 1 • h− 1). PA can be differentiated based on the absolute intensity as follows: very light-intensity PA: 1.6 to 2.0 METs; light-intensity PA: 2.0 to 2.9 METs; moderate-intensity PA: 3.0 to 5.9 METs, vigorous-intensity PA: 6.0 to 8.7 METs, and near-to-maximal intensity: ≥ 8.8 METs[102].
- Sedentary Behaviour (SB)
This is defined as any waking behavior characterized by an energy expenditure of 1.5 METs or lower while sitting, reclining, or lying [103].
- Prolonged sitting
This is a subcategory of sedentary behavior in which an individual typically spends time in a sitting posture ≥ 30 minutes [8].
- Reducing/interrupting prolonged sitting model
refers to interrupting prolonged sitting with physical activity break for alleviating or counteracting the detrimental effects of sedentary behavior lasting for consecutive 60 minutes or greater [8].
References Uncategorized References
- 1.Ludyga S., et al. , Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nature human behaviour, 2020. 4(6): p. 603–612. [DOI] [PubMed] [Google Scholar]
- 2.Stillman CM, et al. , Effects of exercise on brain and cognition across age groups and health states. Trends in neurosciences, 2020. 43(7): p. 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AM, et al. , Differentiating the influence of sedentary behavior and physical activity on brain health in late adulthood. Experimental Gerontology, 2023. 180: p. 112246. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler MJ, et al. , Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 2017. 3(3): p. 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallgren M, Dunstan DW, and Owen N, Passive versus mentally active sedentary behaviors and depression. Exercise and Sport Sciences Reviews, 2020. 48(1): p. 20–27. [DOI] [PubMed] [Google Scholar]
- 6.Tapia-Serrano MA, et al. , Prevalence of meeting 24-Hour Movement Guidelines from pre-school to adolescence: A systematic review and meta-analysis including 387,437 participants and 23 countries. J Sport Health Sci, 2022. 11(4): p. 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong C., et al. , Associations between meeting 24-hour movement guidelines and quality of life among children and adolescents with autism spectrum disorder. J Sport Health Sci, 2023. 12(1): p. 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto AJ, et al. , The Physiology of Sedentary Behavior. Physiological Reviews, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey PC, et al. , Managing sedentary behavior to reduce the risk of diabetes and cardiovascular disease. Current diabetes reports, 2014. 14: p. 1–11. [DOI] [PubMed] [Google Scholar]
- 10.Sallis JF, Owen N, and Fisher E, Ecological models of health behavior. Health behavior: Theory, research, and practice, 2015. 5(43–64). [Google Scholar]
- 11.Dunstan DW, et al. , Too much sitting–a health hazard. Diabetes research and clinical practice, 2012. 97(3): p. 368–376. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan DW, et al. , Sit less and move more for cardiovascular health: emerging insights and opportunities. Nature Reviews Cardiology, 2021. 18(9): p. 637–648. [DOI] [PubMed] [Google Scholar]
- 13.Raichlen DA, et al. , Sitting, squatting, and the evolutionary biology of human inactivity. Proc Natl Acad Sci U S A, 2020. 117(13): p. 7115–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L., et al. , Trends in Sedentary Behavior Among the US Population, 2001-2016. JAMA, 2019. 321(16): p. 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., et al. , Secular trends in sedentary behaviors and associations with weight indicators among Chinese reproductive-age women from 2004 to 2015: findings from the China Health and Nutrition Survey. International Journal of Obesity, 2020. 44(11): p. 2267–2278. [DOI] [PubMed] [Google Scholar]
- 16.Matthews CE, et al. , Sedentary Behavior in U.S. Adults: Fall 2019. Med Sci Sports Exerc, 2021. 53(12): p. 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raichlen DA, et al. , Sedentary Behavior and Incident Dementia Among Older Adults. JAMA, 2023. 330(10): p. 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landhuis CE, et al. , Does Childhood Television Viewing Lead to Attention Problems in Adolescence? Results From a Prospective Longitudinal Study. Pediatrics, 2007. 120(3): p. 532–537. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JG, et al. , Extensive Television Viewing and the Development of Attention and Learning Difficulties During Adolescence. Archives of Pediatrics & Adolescent Medicine, 2007. 161(5): p. 480–486. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X., et al. , Association of Sedentary Time and Physical Activity With Executive Function Among Children. Academic Pediatrics, 2021. 21(1): p. 63–69. [DOI] [PubMed] [Google Scholar]
- 21.Swing EL, et al. , Television and Video Game Exposure and the Development of Attention Problems. Pediatrics, 2010. 126(2): p. 214–221. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson CJ, et al. , Not Worth the Fuss After All? Cross-sectional and Prospective Data on Violent Video Game Influences on Aggression, Visuospatial Cognition and Mathematics Ability in a Sample of Youth. Journal of Youth and Adolescence, 2013. 42(1): p. 109–122. [DOI] [PubMed] [Google Scholar]
- 23.Syväoja HJ, et al. , The associations of objectively measured physical activity and sedentary time with cognitive functions in school-aged children. PLoS ONE, 2014. 9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickel EE, Sedentary Time, Physical Activity, and Executive Function in a Longitudinal Study of Youth. Journal of Physical Activity and Health, 2017. 14(3): p. 222–228. [DOI] [PubMed] [Google Scholar]
- 25.Mora-Gonzalez J., et al. , Fitness, physical activity, sedentary time, inhibitory control, and neuroelectric activity in children with overweight or obesity: The ActiveBrains project. Psychophysiology, 2020. 57(6): p. e13579. [DOI] [PubMed] [Google Scholar]
- 26.Horowitz-Kraus T and Hutton JS, Brain connectivity in children is increased by the time they spend reading books and decreased by the length of exposure to screen-based media. Acta Paediatrica, 2018. 107(4): p. 685–693. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz JR, et al. , Physical activity, fitness, weight status, and cognitive performance in adolescents. J Pediatr, 2010. 157(6): p. 917–922.e1–5. [DOI] [PubMed] [Google Scholar]
- 28.Paulus MP, et al. , Screen media activity and brain structure in youth: Evidence for diverse structural correlation networks from the ABCD study. NeuroImage, 2019. 185: p. 140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi H., et al. , Impact of frequency of internet use on development of brain structures and verbal intelligence: Longitudinal analyses. Human Brain Mapping, 2018. 39(11): p. 4471–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zavala-Crichton JP, et al. , Association of Sedentary Behavior with Brain Structure and Intelligence in Children with Overweight or Obesity: The ActiveBrains Project. Journal of Clinical Medicine, 2020. 9(4): p. 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migueles JH, et al. , Associations of Objectively-Assessed Physical Activity and Sedentary Time with Hippocampal Gray Matter Volume in Children with Overweight/Obesity. Journal of Clinical Medicine, 2020. 9(4): p. 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadenas-Sanchez C., et al. , Physical activity, sedentary time, and fitness in relation to brain shapes in children with overweight/obesity: Links to intelligence. Scandinavian Journal of Medicine & Science in Sports, 2023. 33(3): p. 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi H., et al. , The Impact of Television Viewing on Brain Structures: Cross-Sectional and Longitudinal Analyses. Cerebral Cortex, 2013. 25(5): p. 1188–1197. [DOI] [PubMed] [Google Scholar]
- 34.Munasib A and Bhattacharya S, Is the ‘Idiot's Box’ raising idiocy? Early and middle childhood television watching and child cognitive outcome. Economics of Education Review, 2010. 29(5): p. 873–883. [Google Scholar]
- 35.Wickel EE and Howie EK, Prospective bi-directional associations between sedentary time and physical activity with cognitive performance: a cohort study. Journal of Sports Sciences, 2019. 37(6): p. 630–637. [DOI] [PubMed] [Google Scholar]
- 36.Li L., et al. , Physical Activity and Inhibitory Control: The Mediating Role of Sleep Quality and Sleep Efficiency. Brain Sci, 2021. 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadenas-Sanchez C., et al. , Physical activity, sedentary time, and fitness in relation to brain shapes in children with overweight/obesity: Links to intelligence. Scandinavian Journal of Medicine & Science in Sports, 2023. 33(3): p. 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zavala-Crichton JP and Esteban-Cornejo I, Association of sedentary behavior with brain structure and intelligence in children with overweight or obesity: the ActiveBrains project. Journal of clinical medicine, 2020. 9(4): p. 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West GL, et al. , Playing Super Mario 64 increases hippocampal grey matter in older adults. PloS one, 2017. 12(12): p. e0187779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adelantado-Renau M., et al. , Association Between Screen Media Use and Academic Performance Among Children and Adolescents: A Systematic Review and Meta-analysis. JAMA Pediatrics, 2019. 173(11): p. 1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pindus DM, et al. , The relationships between prolonged sedentary time, physical activity, cognitive control, and P3 in adults with overweight and obesity. International Journal of Obesity, 2021. 45(4): p. 746–757. [DOI] [PubMed] [Google Scholar]
- 42.Felez-Nobrega M., et al. , The association of context-specific sitting time and physical activity intensity to working memory capacity and academic achievement in young adults. European Journal of Public Health, 2017. 27(4): p. 741–746. [DOI] [PubMed] [Google Scholar]
- 43.Hoang TD, et al. , Effect of Early Adult Patterns of Physical Activity and Television Viewing on Midlife Cognitive Function. JAMA Psychiatry, 2016. 73(1): p. 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pindus DM, et al. , Opposing associations between sedentary time and decision-making competence in young adults revealed by functional connectivity in the dorsal attention network. Scientific reports, 2020. 10(1): p. 13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuang J., et al. , Psychometric evaluation of the inventory of dimensions of emerging adulthood (IDEA) in China. International Journal of Clinical and Health Psychology, 2023. 23(1): p. 100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dillon K., et al. , Total sedentary time and cognitive function in middle-aged and older adults: A systematic review and meta-analysis. Sports Medicine-Open, 2022. 8(1): p. 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rojer AG, et al. , Objectively assessed physical activity and sedentary behavior and global cognitive function in older adults: a systematic review. Mechanisms of ageing and development, 2021. 198: p. 111524. [DOI] [PubMed] [Google Scholar]
- 48.Maasakkers CM, et al. , The association of sedentary behaviour and cognitive function in people without dementia: a coordinated analysis across five cohort studies from COSMIC. Sports Medicine, 2020. 50: p. 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falck RS, Davis JC, and Liu-Ambrose T, What is the association between sedentary behaviour and cognitive function? A systematic review. British journal of sports medicine, 2017. 51(10): p. 800–811. [DOI] [PubMed] [Google Scholar]
- 50.Olanrewaju O., et al. , Sedentary behaviours, cognitive function, and possible mechanisms in older adults: a systematic review. Aging clinical and experimental research, 2020. 32: p. 969–984. [DOI] [PubMed] [Google Scholar]
- 51.Falck RS, et al. , Cross-sectional relationships of physical activity and sedentary behavior with cognitive function in older adults with probable mild cognitive impairment. Physical therapy, 2017. 97(10): p. 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan S., et al. , Association between sedentary behavior and the risk of dementia: a systematic review and meta-analysis. Transl Psychiatry, 2020. 10(1): p. 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen S., et al. , Accelerometer-measured physical activity and sitting with incident mild cognitive impairment or probable dementia among older women. Alzheimer's & Dementia, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engeroff T., et al. , Is objectively assessed sedentary behavior, physical activity and cardiorespiratory fitness linked to brain plasticity outcomes in old age? Neuroscience, 2018. 388: p. 384–392. [DOI] [PubMed] [Google Scholar]
- 55.Siddarth P., et al. , Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PloS one, 2018. 13(4): p. e0195549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zlatar ZZ, et al. , Dose-dependent association of accelerometer-measured physical activity and sedentary time with brain perfusion in aging. Experimental gerontology, 2019. 125: p. 110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnardottir NY, et al. , Association of change in brain structure to objectively measured physical activity and sedentary behavior in older adults: Age, Gene/Environment Susceptibility-Reykjavik Study. Behavioural brain research, 2016. 296: p. 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falck RS, et al. , Not just for joints: the associations of moderate-to-vigorous physical activity and sedentary behavior with brain cortical thickness. Medicine & Science in Sports & Exercise, 2020. 52(10): p. 2217–2223. [DOI] [PubMed] [Google Scholar]
- 59.Maasakkers CM, et al. , Hemodynamic and structural brain measures in high and low sedentary older adults. Journal of Cerebral Blood Flow & Metabolism, 2021. 41(10): p. 2607–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erickson KI, et al. , The brain-derived neurotrophic factor Val66Met polymorphism moderates an effect of physical activity on working memory performance. Psychological science, 2013. 24(9): p. 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leckie RL, et al. , BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci, 2014. 8: p. 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makizako H., et al. , Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. J Gerontol A Biol Sci Med Sci, 2015. 70(4): p. 480–6. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto M., et al. , Hippocampal atrophy and memory dysfunction associated with physical inactivity in community-dwelling elderly subjects: The Sefuri study. Brain Behav, 2017. 7(2): p. e00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erickson KI, et al. , Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A, 2011. 108(7): p. 3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wheeler MJ, et al. , Distinct effects of acute exercise and breaks in sitting on working memory and executive function in older adults: a three-arm, randomised cross-over trial to evaluate the effects of exercise with and without breaks in sitting on cognition. British journal of sports medicine, 2020. 54(13): p. 776–781. [DOI] [PubMed] [Google Scholar]
- 66.Wheeler MJ, et al. , Morning exercise mitigates the impact of prolonged sitting on cerebral blood flow in older adults. Journal of applied physiology, 2019. 126(4): p. 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheeler MJ, et al. , Effect of morning exercise with or without breaks in prolonged sitting on blood pressure in older overweight/obese adults: Evidence for sex differences. Hypertension, 2019. 73(4): p. 859–867. [DOI] [PubMed] [Google Scholar]
- 68.Falck RS, et al. , Can we improve cognitive function among adults with osteoarthritis by increasing moderate-to-vigorous physical activity and reducing sedentary behaviour? Secondary analysis of the MONITOR-OA study. BMC musculoskeletal disorders, 2018. 19(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weber A., et al. , Association between osteoarthritis and increased risk of dementia: a systemic review and meta-analysis. Medicine, 2019. 98(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carter SE, et al. , Using an e-health intervention to reduce prolonged sitting in UK office workers: a randomised acceptability and feasibility study. International Journal of Environmental Research and Public Health, 2020. 17(23): p. 8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartman YA, et al. , Long-Term and Acute Benefits of Reduced Sitting on Vascular Flow and Function. Medicine and Science in Sports and Exercise, 2021. 53(2): p. 341–350. [DOI] [PubMed] [Google Scholar]
- 72.Hadgraft NT, et al. , Effects of sedentary behaviour interventions on biomarkers of cardiometabolic risk in adults: systematic review with meta-analyses. British journal of sports medicine, 2021. 55(3): p. 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raz N., et al. , Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex, 2005. 15(11): p. 1676–89. [DOI] [PubMed] [Google Scholar]
- 74.Aunger J and Wagnild J, Objective and subjective measurement of sedentary behavior in human adults: A toolkit. American journal of human biology, 2022. 34(1): p. e23546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurita S., et al. , Development of a Questionnaire to Evaluate Older Adults' Total Sedentary Time and Sedentary Time With Cognitive Activity. J Geriatr Psychiatry Neurol, 2022. 35(3): p. 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prince SA, et al. , A comparison of self-reported and device measured sedentary behaviour in adults: a systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity, 2020. 17(1): p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gardner B., et al. , Is sitting invisible? Exploring how people mentally represent sitting. International Journal of Behavioral Nutrition and Physical Activity, 2019. 16(1): p. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munafò MR, et al. , A manifesto for reproducible science. Nature human behaviour, 2017. 1(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Migueles JH, et al. , GRANADA consensus on analytical approaches to assess associations with accelerometer-determined physical behaviours (physical activity, sedentary behaviour and sleep) in epidemiological studies. British journal of sports medicine, 2022. 56(7): p. 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heesch KC, et al. , Validity of objective methods for measuring sedentary behaviour in older adults: a systematic review. International Journal of Behavioral Nutrition and Physical Activity, 2018. 15(1): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reichert M., et al. , Ambulatory assessment for physical activity research: State of the science, best practices and future directions. Psychology of Sport and Exercise, 2020. 50: p. 101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prince SA, et al. , Measurement of sedentary behaviour in population health surveys: a review and recommendations. PeerJ, 2017. 5: p. e4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hartson KR, et al. , Use of Electronic Ecological Momentary Assessment Methodologies in Physical Activity, Sedentary Behavior, and Sleep Research in Young Adults: Systematic Review. Journal of Medical Internet Research, 2023. 25: p. e46783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Q., et al. , Neurobehavioral mechanisms underlying the effects of physical exercise break on episodic memory during prolonged sitting. Complementary Therapies in Clinical Practice, 2022. 48: p. 101553. [DOI] [PubMed] [Google Scholar]
- 85.Raichlen DA, et al. , Leisure-time sedentary behaviors are differentially associated with all-cause dementia regardless of engagement in physical activity. Proceedings of the National Academy of Sciences, 2022. 119(35): p. e2206931119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanderson E., et al. , Mendelian randomization. Nature Reviews Methods Primers, 2022. 2(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baumeister SE, et al. , Physical activity and risk of Alzheimer disease: A 2-sample mendelian randomization study. Neurology, 2020. 95(13): p. e1897–e1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi KW, et al. , Assessment of Bidirectional Relationships Between Physical Activity and Depression Among Adults: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry, 2019. 76(4): p. 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carter SE, et al. , Are acute sitting-induced changes in inflammation and cerebrovascular function related to impaired mood and cognition? Sport Sciences for Health, 2021. 17(3): p. 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bojsen-Møller E., et al. , The effect of breaking up prolonged sitting on paired associative stimulation-induced plasticity. Experimental Brain Research, 2020. 238(11): p. 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wennberg P., et al. , Acute effects of breaking up prolonged sitting on fatigue and cognition: a pilot study. BMJ Open, 2016. 6(2): p. e009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zou L., et al. , Look into my eyes: What can eye-based measures tell us about the relationship between physical activity and cognitive performance? J Sport Health Sci, 2023. 12(5): p. 568–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Q., et al. , Cognitive benefits of exercise interventions: an fMRI activation likelihood estimation meta-analysis. Brain Struct Funct, 2021. 226(3): p. 601–619. [DOI] [PubMed] [Google Scholar]
- 94.Ashdown-Franks G., et al. , Association of leisure-time sedentary behavior with fast food and carbonated soft drink consumption among 133,555 adolescents aged 12-15 years in 44 low- and middle-income countries. Int J Behav Nutr Phys Act, 2019. 16(1): p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Pan Y, and Li H, What is brain health and why is it important? Bmj, 2020. 371: p. m3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Del Missier F, Mäntylä T, and De Bruin WB, Decision-making competence, executive functioning, and general cognitive abilities. Journal of Behavioral Decision Making, 2012. 25(4): p. 331–351. [Google Scholar]
- 97.Chikhi S, Matton N, and Blanchet S, EEG power spectral measures of cognitive workload: A meta-analysis. Psychophysiology, 2022. 59(6): p. e14009. [DOI] [PubMed] [Google Scholar]
- 98.Polich J., Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology, 2007. 118(10): p. 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glover GH, Overview of functional magnetic resonance imaging. Neurosurg Clin N Am, 2011. 22(2): p. 133–9, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sutton BP, et al. , Current trends and challenges in MRI acquisitions to investigate brain function. Int J Psychophysiol, 2009. 73(1): p. 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ross R., et al. , Canadian 24-Hour Movement Guidelines for Adults aged 18-64 years and Adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab, 2020. 45(10 (Suppl. 2)): p. S57–s102. [DOI] [PubMed] [Google Scholar]
- 102.Caspersen CJ, Powell KE, and Christenson GM, Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep, 1985. 100(2): p. 126–31. [PMC free article] [PubMed] [Google Scholar]
- 103.Tremblay MS, et al. , Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act, 2017. 14(1): p. 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tikhomirova T, Malykh A, and Malykh S, Predicting academic achievement with cognitive abilities: Cross-sectional study across school education. Behavioral sciences, 2020. 10(10): p. 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sánchez-Oliva D., et al. , Sedentary behaviour profiles and longitudinal associations with academic performance in youth: The UP&DOWN study. Journal of Sports Sciences, 2023. 41(2): p. 181–189. [DOI] [PubMed] [Google Scholar]
- 106.Major L., et al. , Domains of sedentary behavior and cognitive function: the health, aging, and body composition study, 1999/2000 to 2006/2007. The Journals of Gerontology: Series A, 2023: p. glad020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raichlen DA, et al. , Leisure-time sedentary behaviors are differentially associated with all-cause dementia regardless of engagement in physical activity. Proceedings of the National Academy of Sciences, 2022. 119(35): p. e2206931119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bakrania K., et al. , Associations Between Sedentary Behaviors and Cognitive Function: Cross-Sectional and Prospective Findings From the UK Biobank. American Journal of Epidemiology, 2017. 187(3): p. 441–454. [DOI] [PubMed] [Google Scholar]
- 109.Kurita S., et al. , Cognitive activity in a sitting position is protectively associated with cognitive impairment among older adults. Geriatrics & Gerontology International, 2019. 19(2): p. 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nguyen L, Murphy K, and Andrews G, Immediate and long-term efficacy of executive functions cognitive training in older adults: A systematic review and meta-analysis. Psychological Bulletin, 2019. 145(7): p. 698–733. [DOI] [PubMed] [Google Scholar]
- 111.Sala G and Gobet F, Cognitive Training Does Not Enhance General Cognition. Trends Cogn Sci, 2019. 23(1): p. 9–20. [DOI] [PubMed] [Google Scholar]
- 112.Erickson KI, et al. , Cognitive aging and the promise of physical activity. Annual Review of Clinical Psychology, 2022. 18: p. 417–442. [DOI] [PubMed] [Google Scholar]
- 113.Halloway S., et al. , Free-living standing activity as assessed by seismic accelerometers and cognitive function in community-dwelling older adults: The MIND trial. The Journals of Gerontology: Series A, 2021. 76(11): p. 1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Erlenbach E, McAuley E, and Gothe NP, The association between light physical activity and cognition among adults: a scoping review. The Journals of Gerontology: Series A, 2021. 76(4): p. 716–724. [DOI] [PubMed] [Google Scholar]
- 115.Sun Y., et al. , Replacement of leisure-time sedentary behavior with various physical activities and the risk of dementia incidence and mortality: A prospective cohort study. Journal of Sport and Health Science, 2023. 12(3): p. 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morris TP, et al. , Brain Structure and Function Predict Adherence to an Exercise Intervention in Older Adults. Med Sci Sports Exerc, 2022. 54(9): p. 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stillman CM and Erickson KI, Physical activity as a model for health neuroscience. Annals of the New York Academy of Sciences, 2018. 1428(1): p. 103–111. [DOI] [PubMed] [Google Scholar]
- 118.Maasakkers CM, et al. , Is there a bidirectional association between sedentary behaviour and cognitive decline in older adults? Findings from the Irish Longitudinal Study on Ageing. Preventive Medicine Reports, 2021. 23: p. 101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference
- 1.Strom RD and Strom PS, Learning throughout life: An intergenerational perspective. 2012: IAP. [Google Scholar]
- 2.Engeroff T., et al. , Is objectively assessed sedentary behavior, physical activity and cardiorespiratory fitness linked to brain plasticity outcomes in old age? Neuroscience, 2018. 388: p. 384–392. [DOI] [PubMed] [Google Scholar]
- 3.Erickson KI, et al. , Cognitive aging and the promise of physical activity. Annual Review of Clinical Psychology, 2022. 18: p. 417–442. [DOI] [PubMed] [Google Scholar]
- 4.Maasakkers CM, et al. , Hemodynamic and structural brain measures in high and low sedentary older adults. Journal of Cerebral Blood Flow & Metabolism, 2021. 41(10): p. 2607–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlatar ZZ, et al. , Dose-dependent association of accelerometer-measured physical activity and sedentary time with brain perfusion in aging. Experimental gerontology, 2019. 125: p. 110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronas UG, et al. , Sedentary Time and White Matter Hyperintensity Volume in Older Adults. Med Sci Sports Exerc, 2019. 51(8): p. 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergoossen LWM, et al. , Association of physical activity and sedentary time with structural brain networks—The Maastricht Study. GeroScience, 2021. 43(1): p. 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrasekaran B., et al. , Does breaking up prolonged sitting improve cognitive functions in sedentary adults? A mapping review and hypothesis formulation on the potential physiological mechanisms. BMC Musculoskelet Disord, 2021. 22(1): p. 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L., et al. , Physical Activity and Inhibitory Control: The Mediating Role of Sleep Quality and Sleep Efficiency. Brain Sci, 2021. 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sewell KR, et al. , Relationships between physical activity, sleep and cognitive function: A narrative review. Neurosci Biobehav Rev, 2021. 130: p. 369–378. [DOI] [PubMed] [Google Scholar]
- 11.Stillman CM, et al. , Effects of exercise on brain and cognition across age groups and health states. Trends in neurosciences, 2020. 43(7): p. 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horowitz-Kraus T and Hutton JS, Brain connectivity in children is increased by the time they spend reading books and decreased by the length of exposure to screen-based media. Acta Paediatrica, 2018. 107(4): p. 685–693. [DOI] [PubMed] [Google Scholar]
- 13.Raichlen DA, et al. , Leisure-time sedentary behaviors are differentially associated with all-cause dementia regardless of engagement in physical activity. Proceedings of the National Academy of Sciences, 2022. 119(35): p. e2206931119. [DOI] [PMC free article] [PubMed] [Google Scholar]


