Abstract
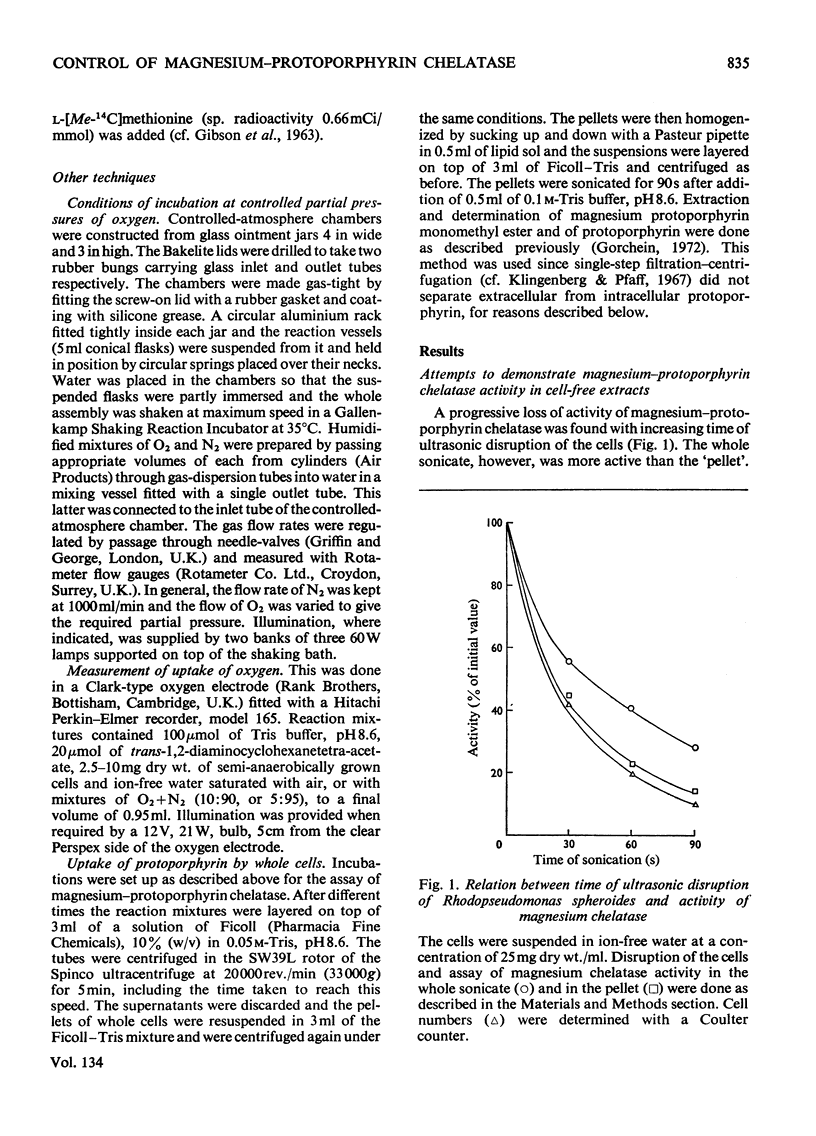
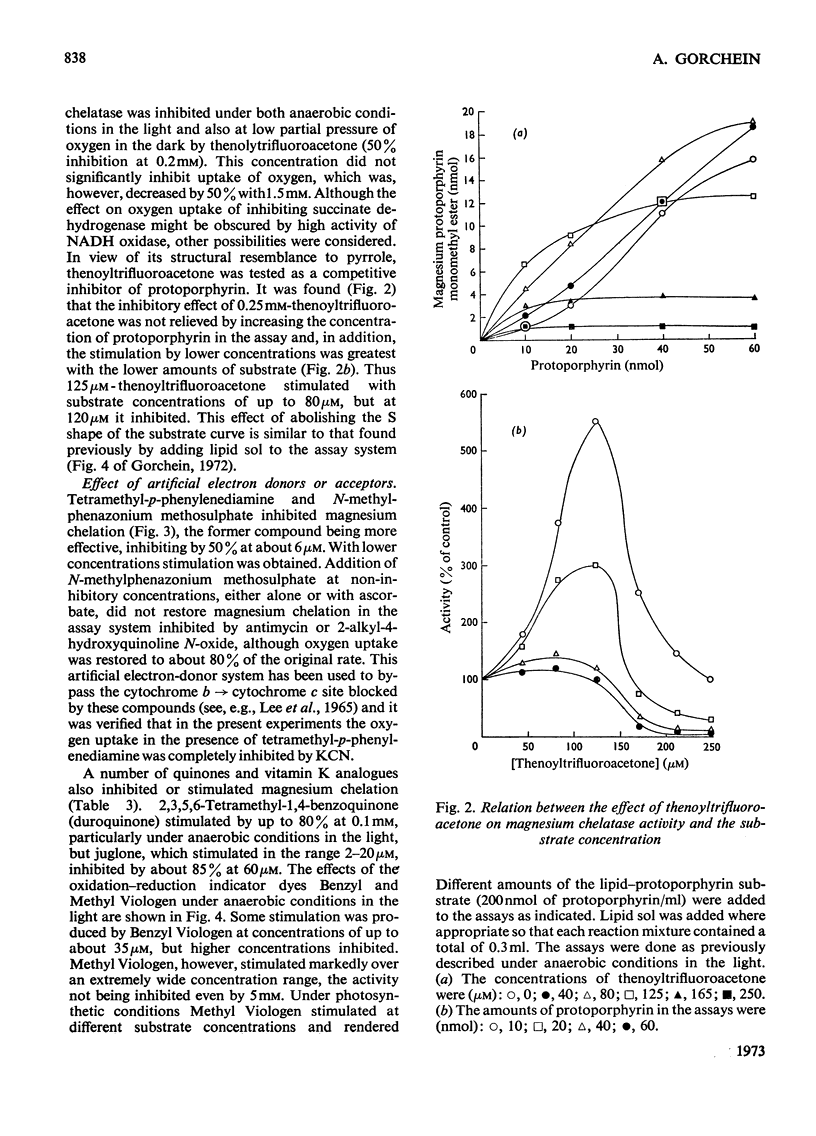
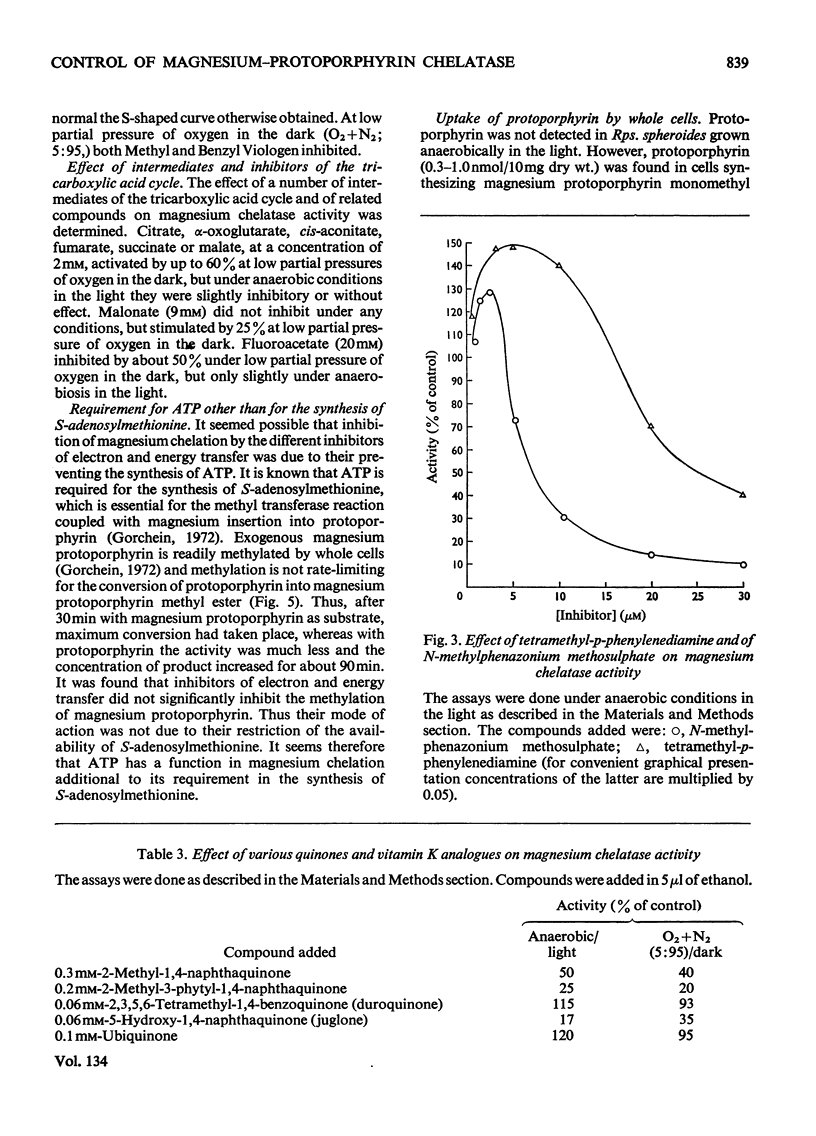
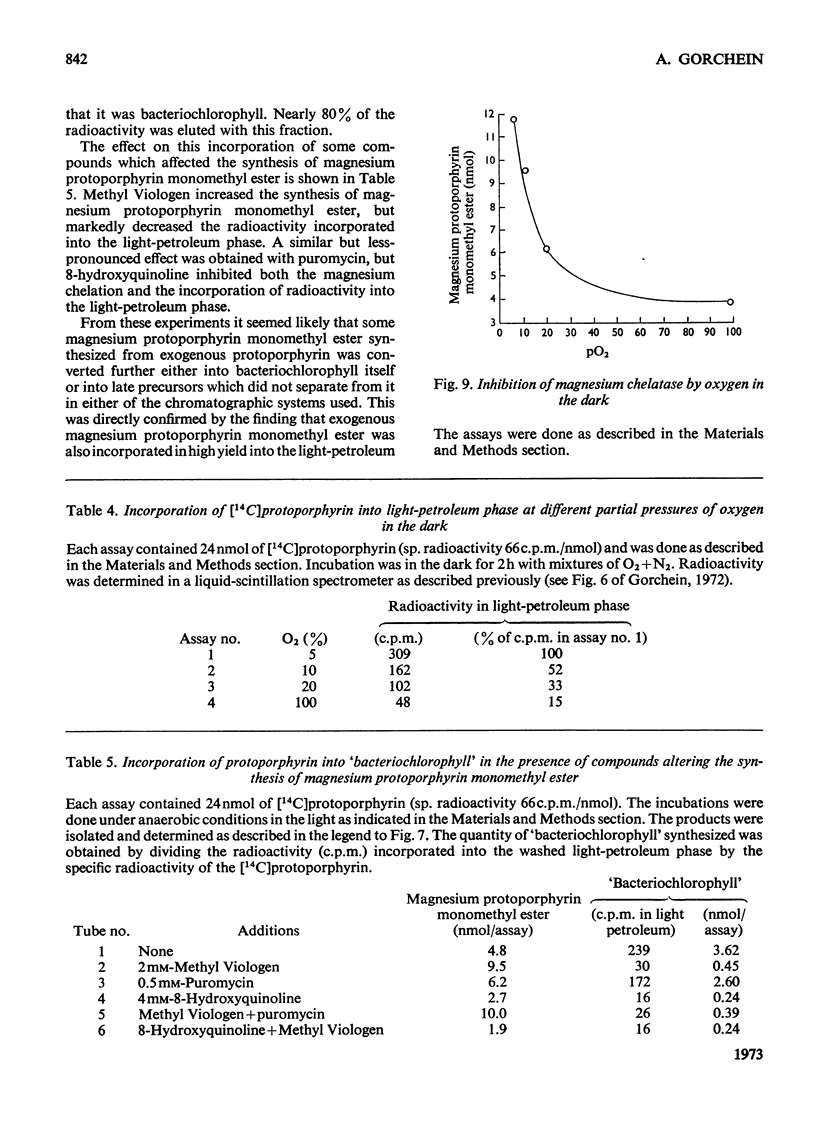
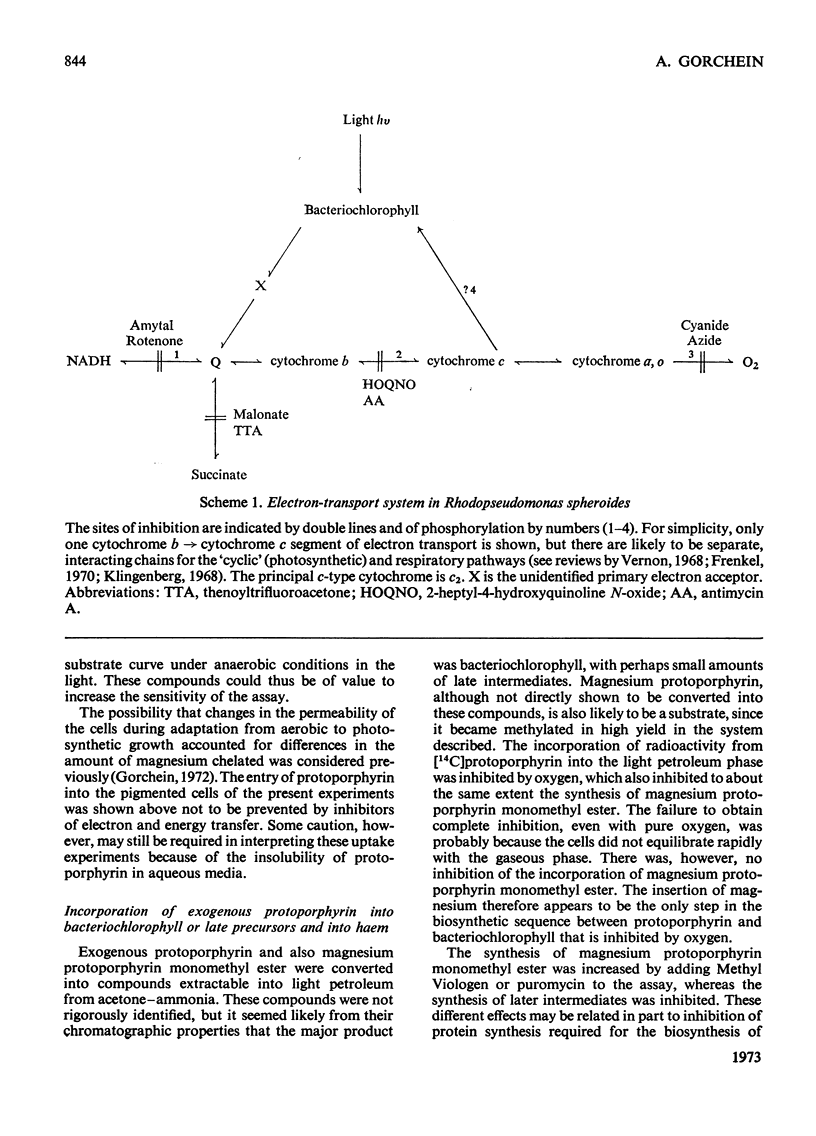
1. Magnesium–protoporphyrin chelatase activity, previously shown in whole cells of Rhodopseudomonas spheroides, could not be demonstrated in cell-free extracts prepared in different ways, although spheroplasts retained moderate activity. Slight activity was detected also in whole cells of Rhodospirillum rubrum. 2. The effects on the activity of the enzyme of inhibitors of electron and energy transfer were studied in whole cells of Rps. spheroides. Amytal, rotenone, azide and cyanide inhibited at low pO2 in the dark but not under anaerobic conditions in the light. Antimycin A and 2-heptyl-4-hydroxyquinoline N-oxide, as well as uncouplers and oligomycin, inhibited under all environmental conditions. 3. The effects on magnesium chelatase activity of intermediates of the tricarboxylic acid cycle, of thenoyltrifluoroacetone, of a number of artificial electron donors or acceptors, of various quinones and of the oxidation–reduction indicator dyes Benzyl Viologen and Methyl Viologen are described. 4. It was concluded that electron transport between a b-type and a c-type cytochrome as well as associated energy conservation and transformation reactions were essential for activity. There was also a specific requirement for ATP. 5. Exogenous protoporphyrin and magnesium protoporphyrin monomethyl ester were incorporated into bacteriochlorophyll or late precursors by whole cells. 6. Evidence is presented that the insertion of magnesium was the only step inhibited by oxygen in the biosynthetic pathway between protoporphyrin and bacteriochlorophyll.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON A. F., CALVIN M. An improved method for the separation and purification of chlorophyll a. Nature. 1962 Apr 21;194:285–286. doi: 10.1038/194285a0. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- DE MATTEIS F., PRIOR B. E. Experimental hepatic porphyria caused by feeding 3,5-diethoxycarbonyl-1,4-dihydro-2,4,6-trimethylpyridine. Comparison with sedormid porphyria. Biochem J. 1962 Apr;83:1–8. doi: 10.1042/bj0830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel A. W. Multiplicity of electron transport reactions in bacterial photosynthesis. Biol Rev Camb Philos Soc. 1970 Nov;45(4):569–616. doi: 10.1111/j.1469-185x.1970.tb01177.x. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDEN M. Separation by paper chromatography of chlorophylls a and b and some of their breakdown products. Biochim Biophys Acta. 1962 Jan 29;56:378–379. doi: 10.1016/0006-3002(62)90584-x. [DOI] [PubMed] [Google Scholar]
- KEHL R., STICH W. Die papierchromatographische Analyse der Porphyrine. Hoppe Seylers Z Physiol Chem. 1951;289(1):6–10. [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- ONISAWA J., LABBE R. F. Effects of diethyl-1, 4-dihydro-2, 4,6-trimethylpyridine-3,5-dicarboxylate on the metabolism of porphyrins and iron. J Biol Chem. 1963 Feb;238:724–727. [PubMed] [Google Scholar]
- STRAAT P. A., NASON A. CHARACTERIZATION OF A NITRATE REDUCTASE FROM THE CHEMOAUTOTROPH NITROBACTER AGILIS. J Biol Chem. 1965 Mar;240:1412–1426. [PubMed] [Google Scholar]
- Vernon L. P. Photochemical and electron transport reactions of bacterial photosynthesis. Bacteriol Rev. 1968 Sep;32(3):243–261. doi: 10.1128/br.32.3.243-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


