Abstract
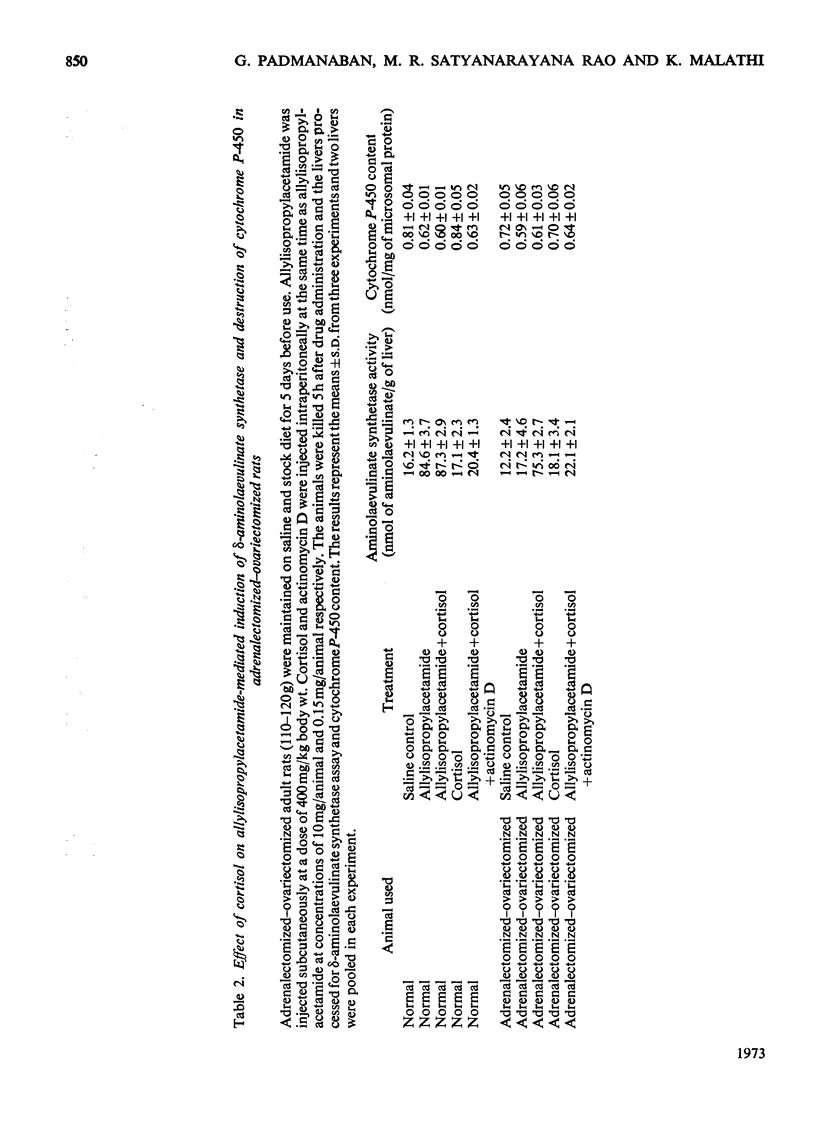
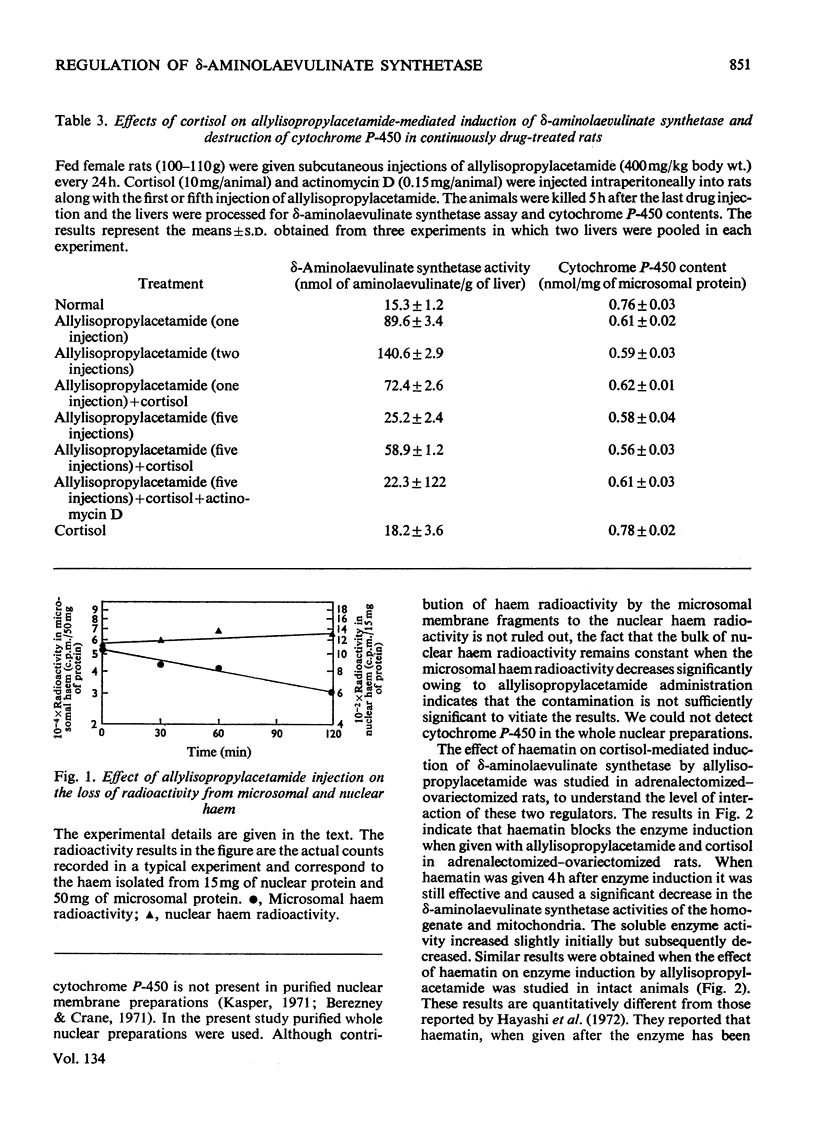
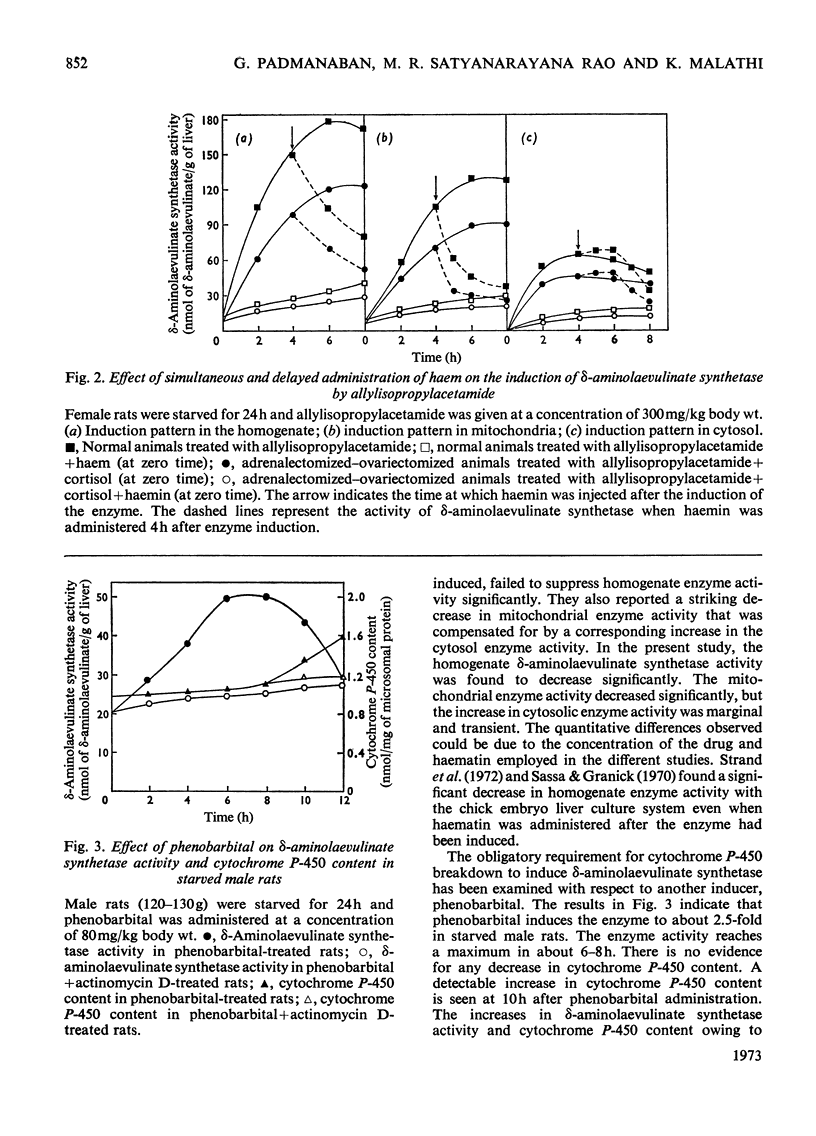
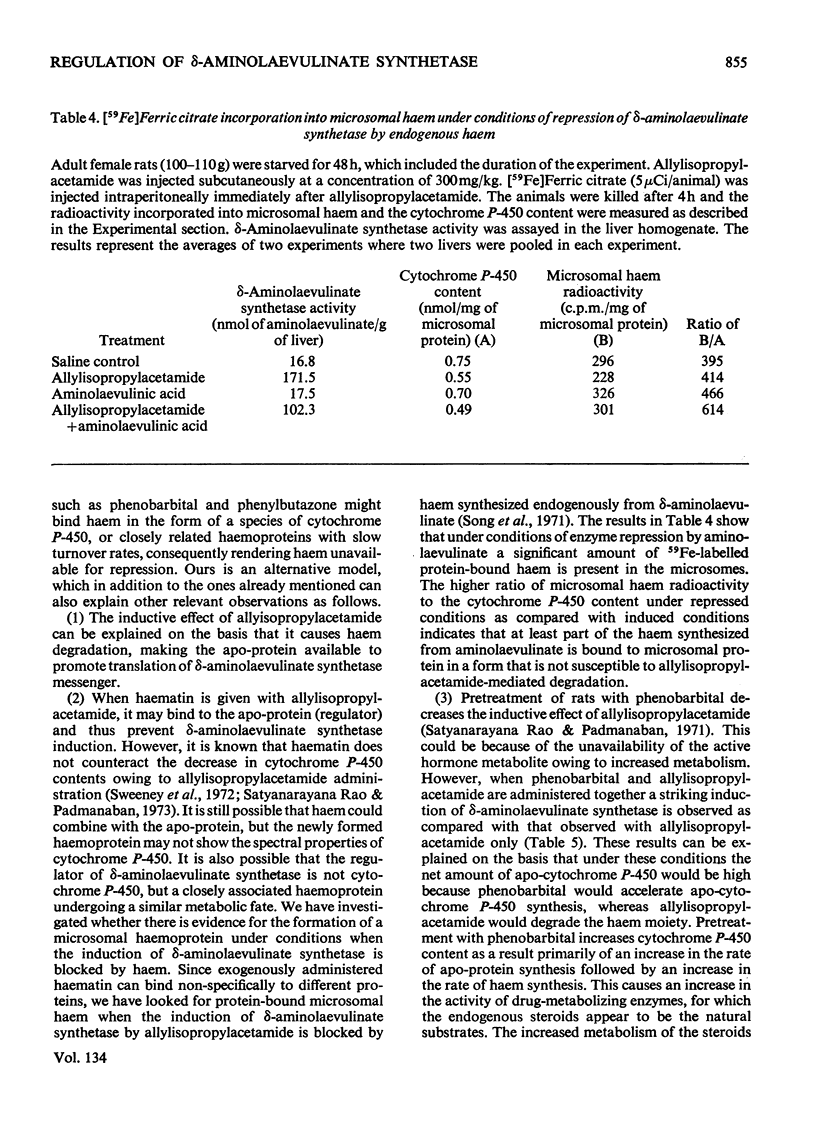
A reciprocal relationship exists between the cytochrome P-450 content and δ-aminolaevulinate synthetase activity in adult rats. In young rats the basal δ-aminolaevulinate synthetase activity is higher and the cytochrome P-450 content is lower compared with the adult rat liver. Administration of allylisopropylacetamide neither induces the enzyme nor causes degradation of cytochrome P-450 in the young rat liver, unlike adult rat liver. Allylisopropylacetamide fails to induce δ-aminolaevulinate synthetase in adrenalectomized–ovariectomized animals or intact animals pretreated with successive doses of the drug, in the absence of cortisol. The cortisol-mediated induction of the enzyme is sensitive to actinomycin D. Allylisopropylacetamide administration degrades microsomal haem but not nuclear haem. Haem does not counteract the decrease in cytochrome P-450 content caused by allylisopropylacetamide administration, but there is evidence for the formation of drug-resistant protein-bound haem in liver microsomal material under these conditions. Phenobarbital induces δ-aminolaevulinate synthetase under conditions when there is no breakdown of cytochrome P-450. On the basis of these results and those already published, a model is proposed for the regulation of δ-aminolaevulinate synthetase induction in rat liver.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin S. D., Palmer E. D., English P. D., Morgan B., Cawthorne M. A., Green J. The role of cytochrome P-450 in cholesterol biogenesis and catabolism. Biochem J. 1972 Jun;128(2):237–242. doi: 10.1042/bj1280237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J., Tephly T. R. Effect of 3-amino-1,2,4-triazole on the stimulation of hepatic microsomal heme synthesis and induction of hepatic microsomal oxidases produced by phenobarbital. Mol Pharmacol. 1969 Jan;5(1):10–20. [PubMed] [Google Scholar]
- Baron J., Tephly T. R. Further studies on the relationship of the stimulatory effects of phenobarbital and 3,4-benzpyrene on hepatic heme synthesis to their effects on hepatic microsomal drug oxidations. Arch Biochem Biophys. 1970 Aug;139(2):410–420. doi: 10.1016/0003-9861(70)90494-7. [DOI] [PubMed] [Google Scholar]
- Baron J., Tephly T. R. The role of heme synthesis during the induction of hepatic microsomal cytochrome P-450 and drug metabolism produced by benzpyrene. Biochem Biophys Res Commun. 1969 Aug 15;36(4):526–532. doi: 10.1016/0006-291x(69)90336-2. [DOI] [PubMed] [Google Scholar]
- Berezney R., Crane F. L. Cytochromes in bovine liver nuclear membranes. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1017–1023. doi: 10.1016/0006-291x(71)90563-8. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. Stimulation of liver 5-aminolaevulinate synthetase by drugs and its relevance to drug-induced accumulation of cytochrome P-450. Studies with phenylbutazone and 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Biochem J. 1972 Mar;126(5):1149–1160. doi: 10.1042/bj1261149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Rapid loss of cytochrome P-450 and haem caused in the liver microsomes by the porphyrogenic agent 2-allyl-2-isopropylacetamide. FEBS Lett. 1970 Feb 25;6(4):343–345. doi: 10.1016/0014-5793(70)80094-1. [DOI] [PubMed] [Google Scholar]
- Gayathri A. K., Rao M. R., Padmanaban G. Studies on the induction of -aminolevulinic acid synthetase iun mouse liver. Arch Biochem Biophys. 1973 Apr;155(2):299–306. doi: 10.1016/0003-9861(73)90118-5. [DOI] [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid control of porphyrin and heme biosynthesis: a new biological function of steroid hormone metabolites. Proc Natl Acad Sci U S A. 1967 May;57(5):1463–1467. doi: 10.1073/pnas.57.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Greim H., Schenkman J. B., Klotzbücher M., Remmer H. The influence of phenobarbital on the turnover of hepatic microsomal cytochrome b5 and cytochrome P-450 hemes in the rat. Biochim Biophys Acta. 1970 Jan 27;201(1):20–25. doi: 10.1016/0304-4165(70)90005-x. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Kurashima Y., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. V. Mechanism of regulation by hemin of the level of -aminolevulinate synthetase in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):10–21. doi: 10.1016/0003-9861(72)90109-9. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. IV. Accumulation of the enzyme in the soluble fraction of rat liver. Arch Biochem Biophys. 1969 Apr;131(1):83–91. doi: 10.1016/0003-9861(69)90107-6. [DOI] [PubMed] [Google Scholar]
- Kappas A., Granick S. Steroid induction of porphyrin synthesis in liver cell culture. II. The effects of heme, uridine diphosphate glucuronic acid, and inhibitors of nucleic acid and protein synthesis on the induction process. J Biol Chem. 1968 Jan 25;243(2):346–351. [PubMed] [Google Scholar]
- Kasper C. B. Biochemical distinctions between the nuclear and microsomal membranes from rat hepatocytes. J Biol Chem. 1971 Feb 10;246(3):577–581. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landaw S. A., Callahan E. W., Jr, Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970 May;49(5):914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin W., Jacobson M., Kuntzman R. Incorporation of radioactive- -aminolevulinic acid into microsomal cytochrome P 450 : selective breakdown of the hemoprotein by allylisopropylacetamide and carbon tetrachloride. Arch Biochem Biophys. 1972 Jan;148(1):262–269. doi: 10.1016/0003-9861(72)90140-3. [DOI] [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Marver H. S., Schmid R., Schützel H. Heme and methemoglobin: naturally occurring repressors of microsomal cytochrome. Biochem Biophys Res Commun. 1968 Dec 30;33(6):969–974. doi: 10.1016/0006-291x(68)90408-7. [DOI] [PubMed] [Google Scholar]
- Matsuoka T., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. 3. Effects of triiodothyronine and hydrocortisone on the induction process. Arch Biochem Biophys. 1968 Aug;126(2):530–538. doi: 10.1016/0003-9861(68)90438-4. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Marver H. S. Chemically induced porphyria: increased microsomal heme turnover after treatment with allylisopropylacetamide. Science. 1971 Jan 8;171(3966):64–66. doi: 10.1126/science.171.3966.64. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Rao M. R., Malathi K., Padmanaban G. The relationship between delta-aminolaevulinate synthetase induction and the concentration of cytochrome P-450 and catalase in rat liver. Biochem J. 1972 Apr;127(3):553–559. doi: 10.1042/bj1270553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. R., Padmanaban G. Biochemical effects of the porphyrinogenic drug allylisopropylacetamide. A comparative study with phenobarbital. Biochem J. 1973 Aug;134(4):859–868. doi: 10.1042/bj1340859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana Rao M. R., Padmanaban G. delta-aminolaevulate synthetase induction and catalase repression by porphyrinogenic drugs. Biochem J. 1971 May;122(4):593–595. doi: 10.1042/bj1220593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. S., Moses H. L., Rosenthal A. S., Gelb N. A., Kappas A. The influence of postnatal development on drug-induced hepatic porphyria and the synthesis of cytochrome P-450. A biochemical and morphological study. J Exp Med. 1971 Nov 1;134(5):1349–1371. doi: 10.1084/jem.134.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Manning J., Marver H. S. The induction of -aminolevulinic acid synthetase in cultured liver cells. The effects of end product and inhibitors of heme synthesis. J Biol Chem. 1972 May 10;247(9):2820–2827. [PubMed] [Google Scholar]
- Sweeney G., Freeman K. B., Rothwell D., Lai H. Decrease in hepatic cytochrome P-450 and catalase following allylisopropyl acetamide: the effect of concomitant hemin administration. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1366–1371. doi: 10.1016/0006-291x(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Marks G. S. Drug-induced porphyrin biosynthesis. V. Effect of protohemin on the transcriptional and post-transcriptional phases of -aminolevulinic acid synthetase induction. Biochem Pharmacol. 1972 Aug 1;21(15):2077–2093. doi: 10.1016/0006-2952(72)90161-x. [DOI] [PubMed] [Google Scholar]
- Waxman A. D., Collins A., Tschudy D. P. Oscillations of hepatic delta-aminolevulinic acid synthetase produced in vivo by heme. Biochem Biophys Res Commun. 1966 Sep 8;24(5):675–683. doi: 10.1016/0006-291x(66)90377-9. [DOI] [PubMed] [Google Scholar]


