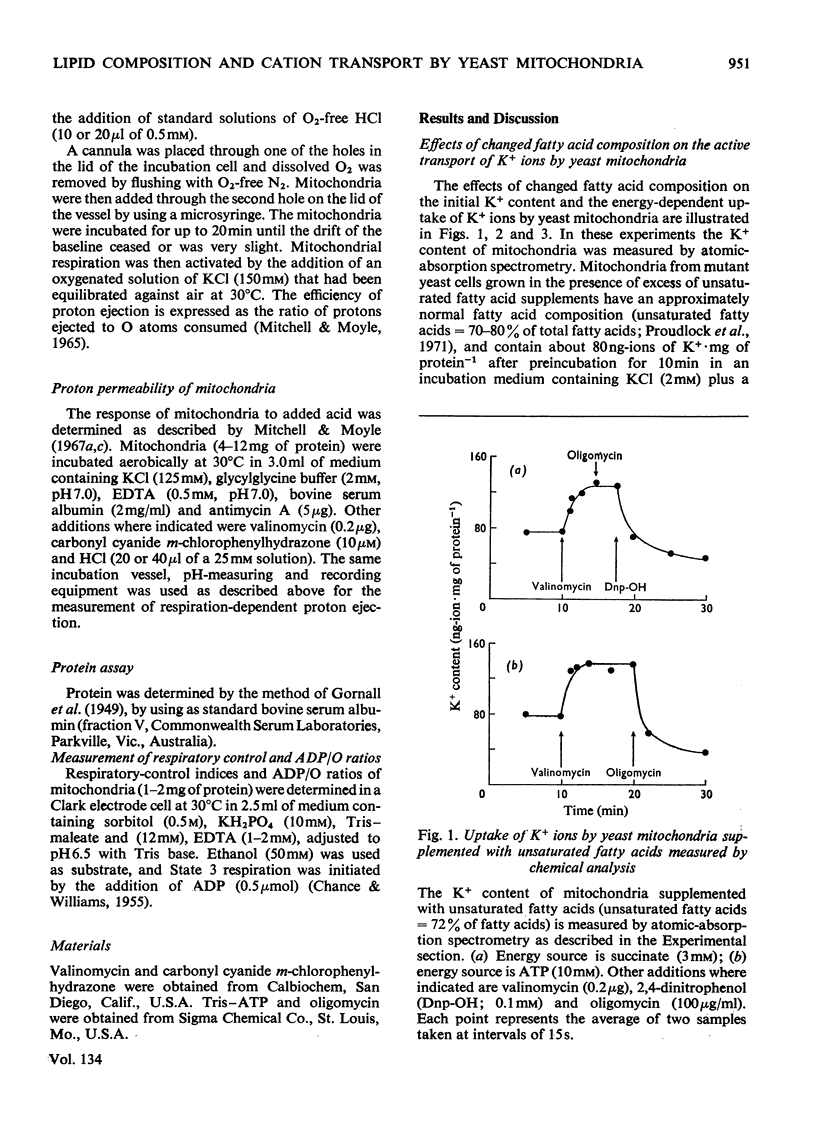
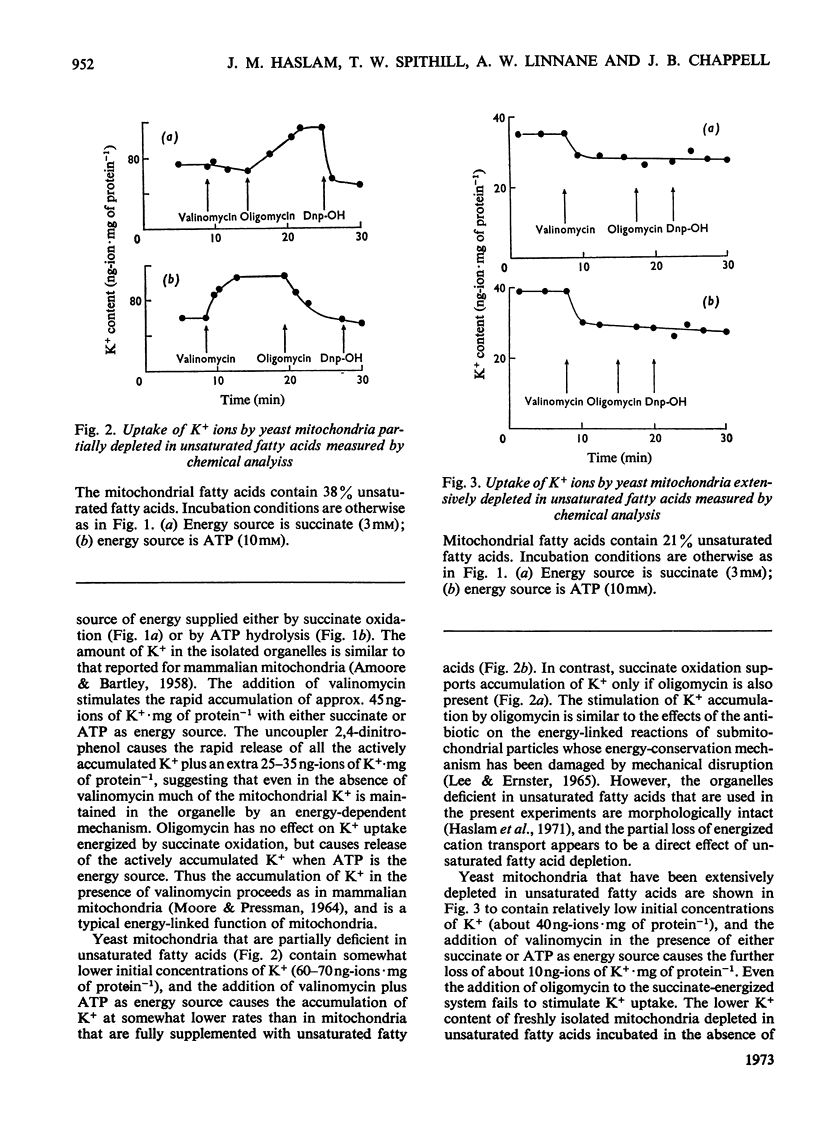
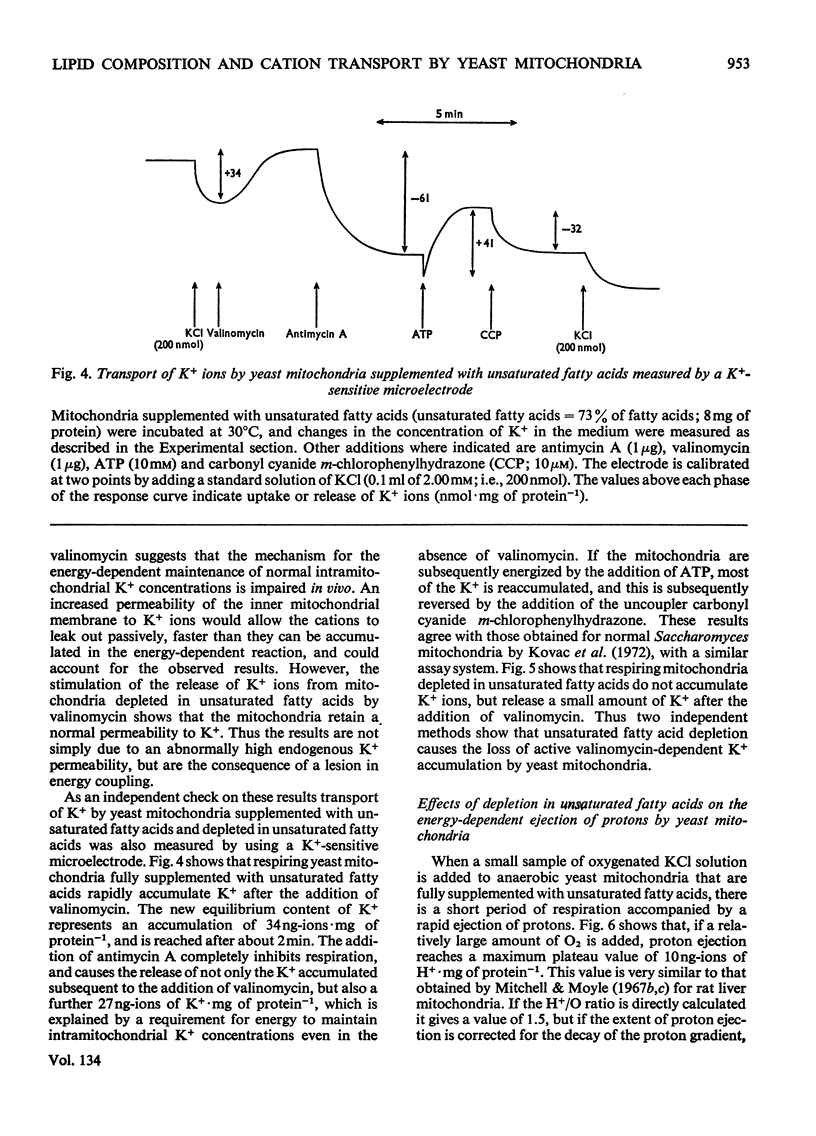
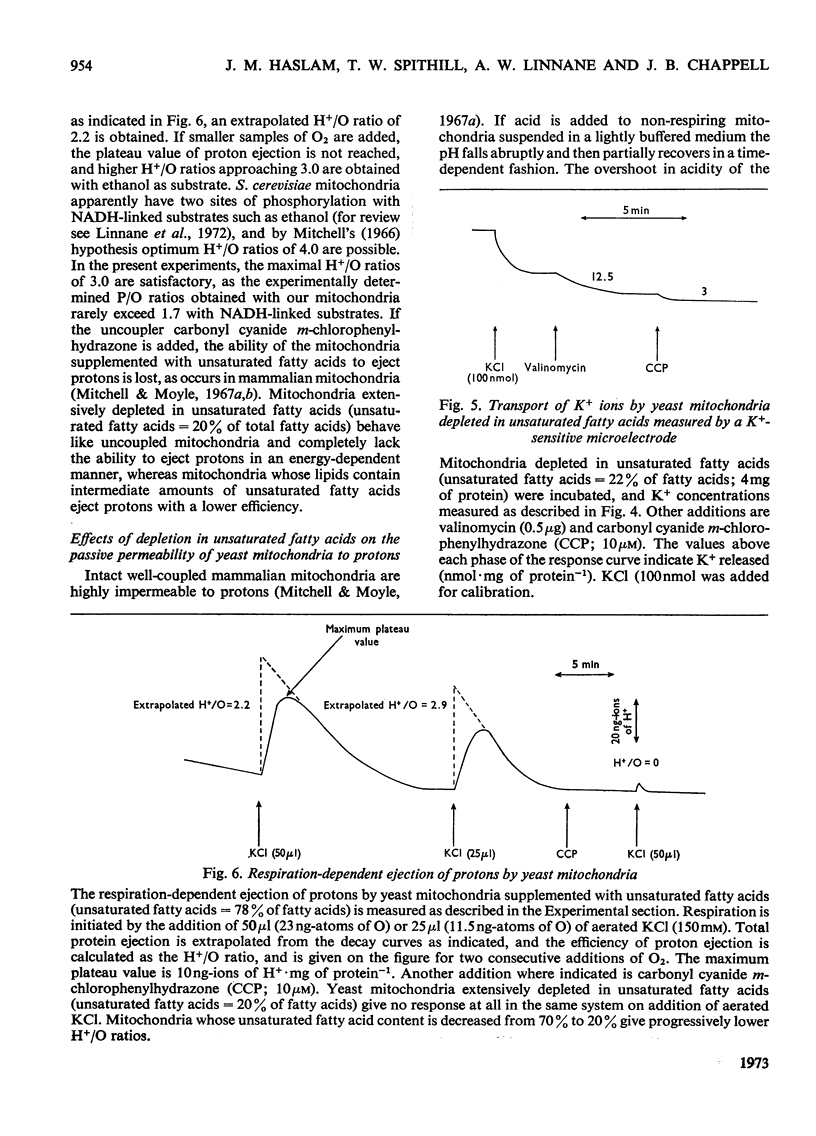
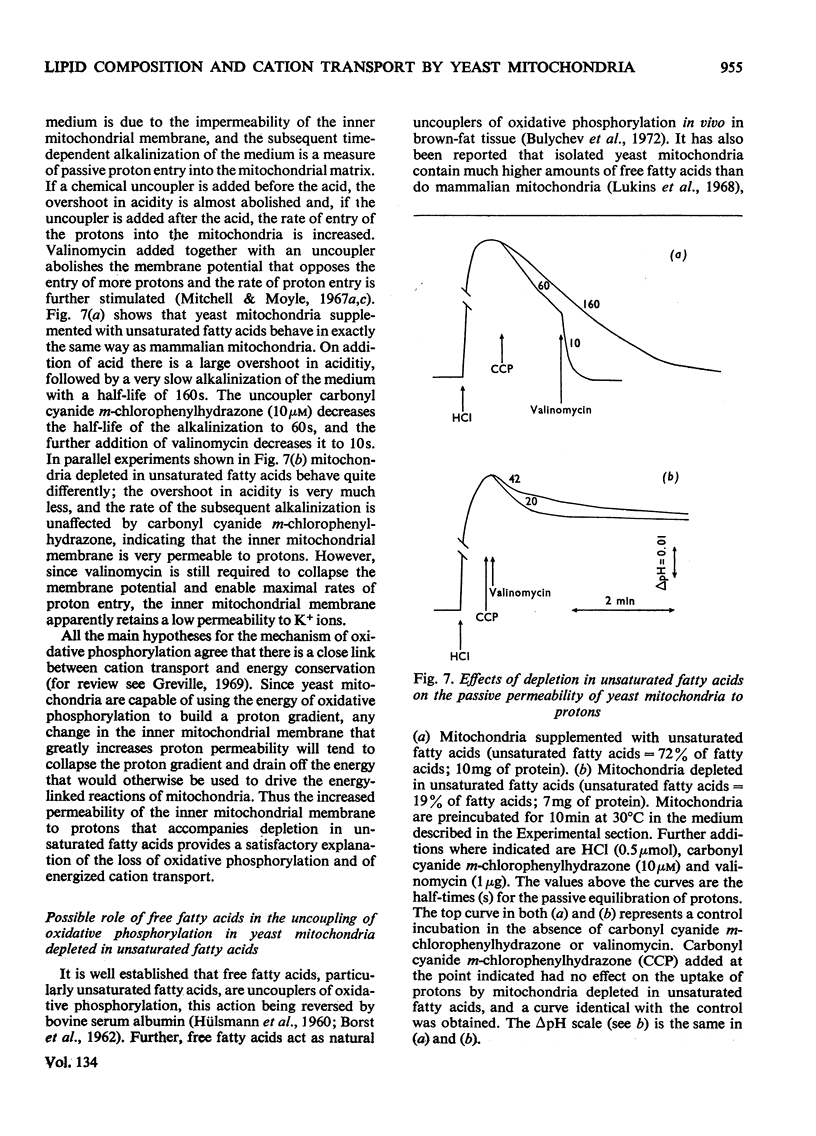
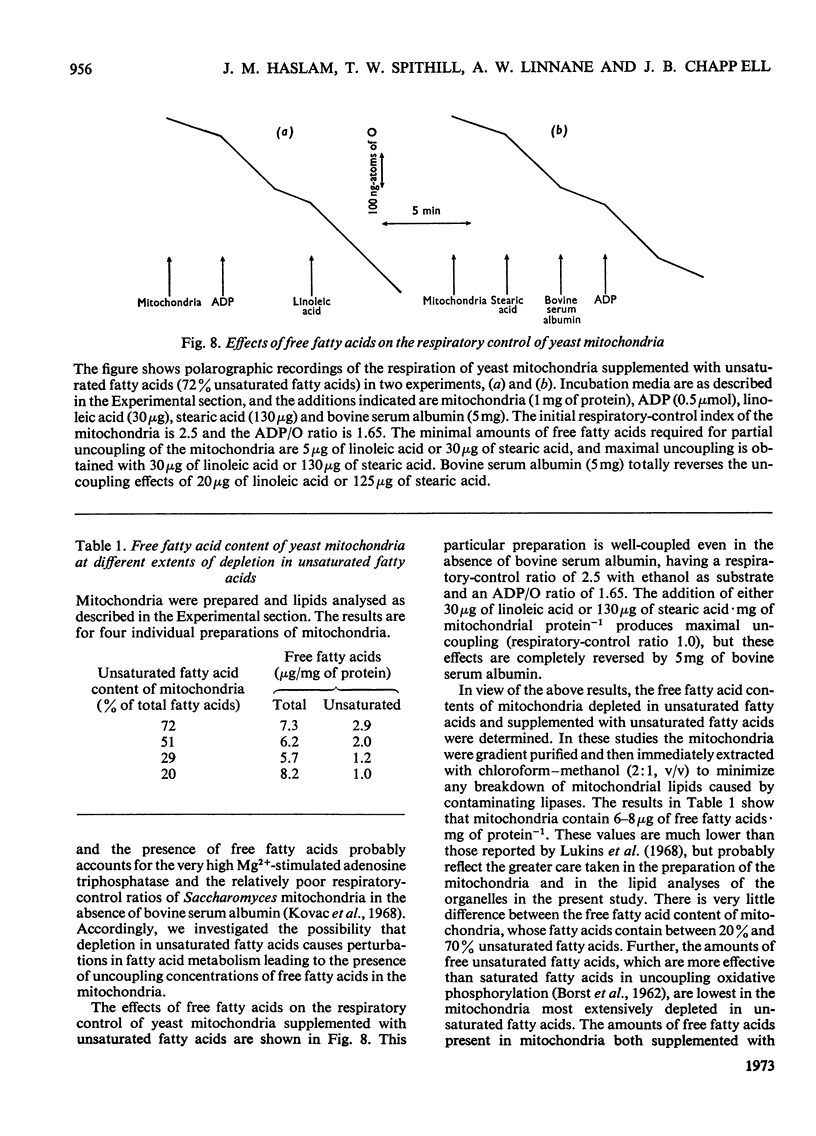
Abstract
1. The fatty acid composition of the membrane lipids of a fatty acid desaturase mutant of Saccharomyces cerevisiae was manipulated by growing the organism in a medium containing defined fatty acid supplements. 2. Mitochondria were obtained whose fatty acids contain between 20% and 80% unsaturated fatty acids. 3. Mitochondria with high proportions of unsaturated fatty acids in their lipids have coupled oxidative phosphorylation with normal P/O ratios, accumulate K+ ions in the presence of valinomycin and an energy source, and eject protons in an energy-dependent fashion. 4. If the unsaturated fatty acid content of the mitochondrial fatty acids is lowered to 20%, the mitochondria simultaneously lose active cation transport and the ability to couple phosphorylation to respiration. 5. The loss of energy-linked reactions is accompanied by an increased passive permeability of the mitochondria to protons. 6. Free fatty acids uncouple oxidative phosphorylation in yeast mitochondria and the effect is reversed by bovine serum albumin. 7. The free fatty acid contents of yeast mitochondria are unaffected by depletion of unsaturated fatty acids, and free fatty acids are not responsible for the uncoupling of oxidative phosphorylation in organelles depleted in unsaturated fatty acids. 8. It is suggested that the loss of energy-linked reactions in yeast mitochondria that are depleted in unsaturated fatty acids is a consequence of the increased passive permeability to protons, and is caused by a change in the physical properties of the lipid phase of the inner mitochondrial membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOORE J. E., BARTLEY W. The permeability of isolated rat-liver mitochondria to sucrose, sodium chloride and potassium chloride at 0 degrees. Biochem J. 1958 Jun;69(2):223–236. doi: 10.1042/bj0690223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Bulychev A., Kramar R., Drahota Z., Lindberg O. Role of a specific endogenous fatty acid fraction in the coupling-uncoupling mechanism of oxidative phosphorylation of brown adipose tissue. Exp Cell Res. 1972 May;72(1):169–187. doi: 10.1016/0014-4827(72)90579-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- HULSMANN W. C., ELLIOTT W. B., SLATER E. C. The nature and mechanism of action of uncoupling agents present in mitochrome preparations. Biochim Biophys Acta. 1960 Apr 8;39:267–276. doi: 10.1016/0006-3002(60)90163-3. [DOI] [PubMed] [Google Scholar]
- Haslam J. M., Perkins M., Linnane A. W. Biogenesis of mitochondria. A requirement for mitochondrial protein synthesis for the formation of a normal adenine nucleotide transporter in yeast mitochondria. Biochem J. 1973 Aug;134(4):935–947. doi: 10.1042/bj1340935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam J. M., Proudlock J. W., Linnane A. W. Biogenesis of mitochondria. 20. The effects of altered membrane lipid composition on mitochondrial oxidative phosphorylation in Saccharomyces cerevisiae. J Bioenerg. 1971 Dec;2(5):351–370. doi: 10.1007/BF01963830. [DOI] [PubMed] [Google Scholar]
- Kovac L., Groot G. S., Racker E. Translocation of protons and potassium ions across the mitochondrial membrane of respiring and respiration-deficient yeasts. Biochim Biophys Acta. 1972 Jan 21;256(1):55–65. doi: 10.1016/0005-2728(72)90162-4. [DOI] [PubMed] [Google Scholar]
- LEE C. P., ERNSTER L. RESTORATION OF OXIDATIVE PHOSPHORYLATION IN NON-PHOSPHORYLATING SUBMITOCHONDRIAL PARTICLES BY OLIGOMYCIN. Biochem Biophys Res Commun. 1965 Feb 17;18:523–529. doi: 10.1016/0006-291x(65)90785-0. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- Lukins H. B., Jollow D., Wallace P. G., Linnane A. W. The biogenesis of mitochondria. 7. The effects of glucose repression on the lipid and enzyme composition of mitochondria from yeast. Aust J Exp Biol Med Sci. 1968 Dec;46(6):651–665. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Stoichiometry of proton translocation through the respiratory chain and adenosine triphosphatase systems of rat liver mitochondria. Nature. 1965 Oct 9;208(5006):147–151. doi: 10.1038/208147a0. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. 19. The effects of unsaturated fatty acid depletion on the lipid composition and energy metabolism of a fatty acid desaturase mutant of Saccharomyces cerevisiae. J Bioenerg. 1971 Dec;2(5):327–349. doi: 10.1007/BF01963829. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Haslam J. M., Linnane A. W. Specific effect of unsaturated fatty acid depletion on mitochondrial oxidative phosphorylation in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1969 Nov 20;37(5):847–852. doi: 10.1016/0006-291x(69)90969-3. [DOI] [PubMed] [Google Scholar]
- Watson K., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. 13. The isolation of mitochondrial structures from anaerobically grown Saccharomyces cerevisiae. J Cell Biol. 1970 Jul;46(1):88–96. doi: 10.1083/jcb.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]


