Abstract
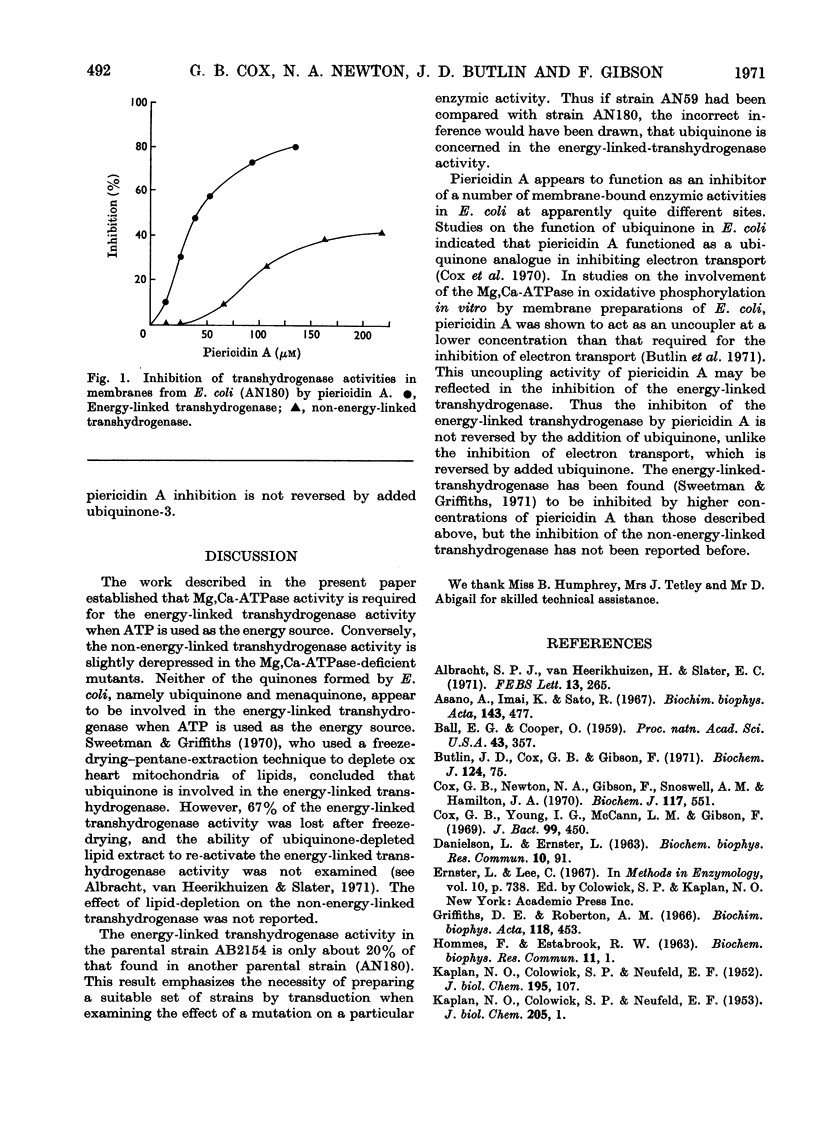
1. Energy-linked and non-energy-linked transhydrogenase activities were assayed in membrane preparations from normal Escherichia coli K 12 and from various mutant strains. 2. The energy-linked transhydrogenase, which uses ATP as energy source, was dependent for activity on the presence of a functional Mg2++Ca2+-stimulated adenosine triphosphatase. 3. Neither of the quinones formed by E. coli, namely ubiquinone-8 and menaquinone-8, was required for normal ATP-dependent energy-linked transhydrogenase activity. 4. The energy-linked transhydrogenase was inhibited by piericidin A at a site unrelated to the sites of inhibition of the electron-transport chain by piericidin A.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albracht S. P.J., Van Heerikhuizen H., Slater E. C. Succinate oxidase activity in the absence of ubiquinone. FEBS Lett. 1971 Mar 22;13(5):265–266. doi: 10.1016/0014-5793(71)80236-3. [DOI] [PubMed] [Google Scholar]
- Asano A., Imai K., Sato R. Oxidative phosphorylation in Micrococcus dentrificans. II. The properties of pyridine nucleotide transhydrogenase. Biochim Biophys Acta. 1967;143(3):477–486. doi: 10.1016/0005-2728(67)90053-9. [DOI] [PubMed] [Google Scholar]
- Ball E. G., Cooper O. THE OXIDATION OF REDUCED TRIPHOSPHOPYRIDINE NUCLEOTIDE AS MEDIATED BY THE TRANSHYDROGENASE REACTION AND ITS INHIBITION BY THYROXINE. Proc Natl Acad Sci U S A. 1957 May 15;43(5):357–364. doi: 10.1073/pnas.43.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Young I. G., McCann L. M., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: location of genes affecting the metabolism of 3-octaprenyl-4-hydroxybenzoic acid and 2-octaprenylphenol. J Bacteriol. 1969 Aug;99(2):450–458. doi: 10.1128/jb.99.2.450-458.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D. E., Roberton A. M. Energy-linked reactions in mitochondria: studies on the mechanism of the energy-linked transhydrogenase reaction. Biochim Biophys Acta. 1966 Jun 15;118(3):453–464. doi: 10.1016/s0926-6593(66)80089-9. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F. Pyridine nucleotide transhydrogenase. II. Direct evidence for and mechanism of the transhydrogenase reaction. J Biol Chem. 1952 Mar;195(1):107–119. [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F. Pyridine nucleotide transhydrogenase. III. Animal tissue transhydrogenases. J Biol Chem. 1953 Nov;205(1):1–15. [PubMed] [Google Scholar]
- KINSKY S. C. Hydrogen oxidation by Clostridium kluyveri. J Biol Chem. 1959 Apr;234(4):973–978. [PubMed] [Google Scholar]
- Keister D. L., Yike N. J. Studies on an energy-lined pyridine nucleotide transhydrogenase in photosynthetic bacteria. I. Demonstration of the reaction in Rhodospirillum rubrum. Biochem Biophys Res Commun. 1966 Aug 23;24(4):519–525. doi: 10.1016/0006-291x(66)90350-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- MURTHY P. S., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XV. REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-LINKED PHOSPHORYLATION. J Biol Chem. 1964 Dec;239:4292–4297. [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Orlando J. A., Sabo D., Curnyn C. Photoreduction of Pyridine Nucleotide by Subcellular Preparations from Rhodopseudomonas spheroides. Plant Physiol. 1966 Jun;41(6):937–945. doi: 10.1104/pp.41.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman A. J., Griffiths D. E. Energy-linked reactions in mitochondria: A requirement for ubiquinone after pentane extraction. FEBS Lett. 1970 Sep 24;10(2):92–96. doi: 10.1016/0014-5793(70)80424-0. [DOI] [PubMed] [Google Scholar]
- Sweetman A. J., Griffiths D. E. Studies on energy-linked reactions. Energy-linked transhydrogenase reaction in Escherichia coli. Biochem J. 1971 Jan;121(1):125–130. doi: 10.1042/bj1210125. [DOI] [PMC free article] [PubMed] [Google Scholar]


