Abstract
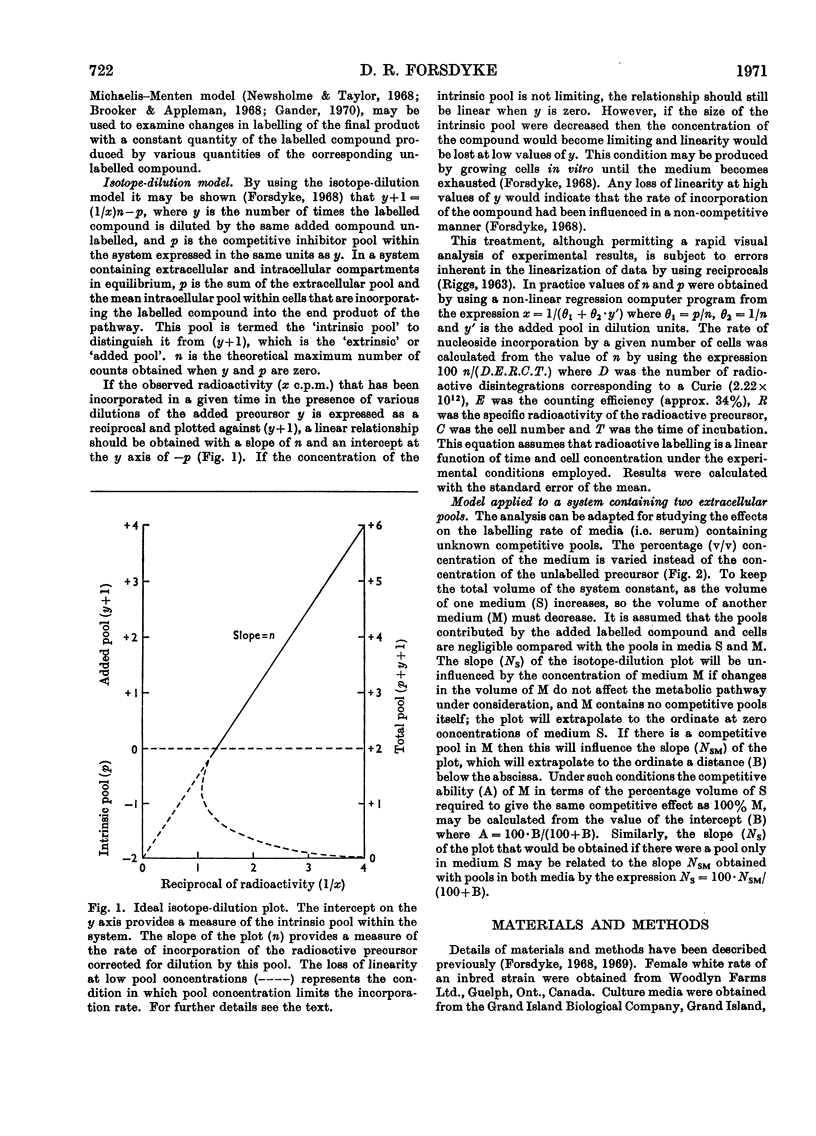
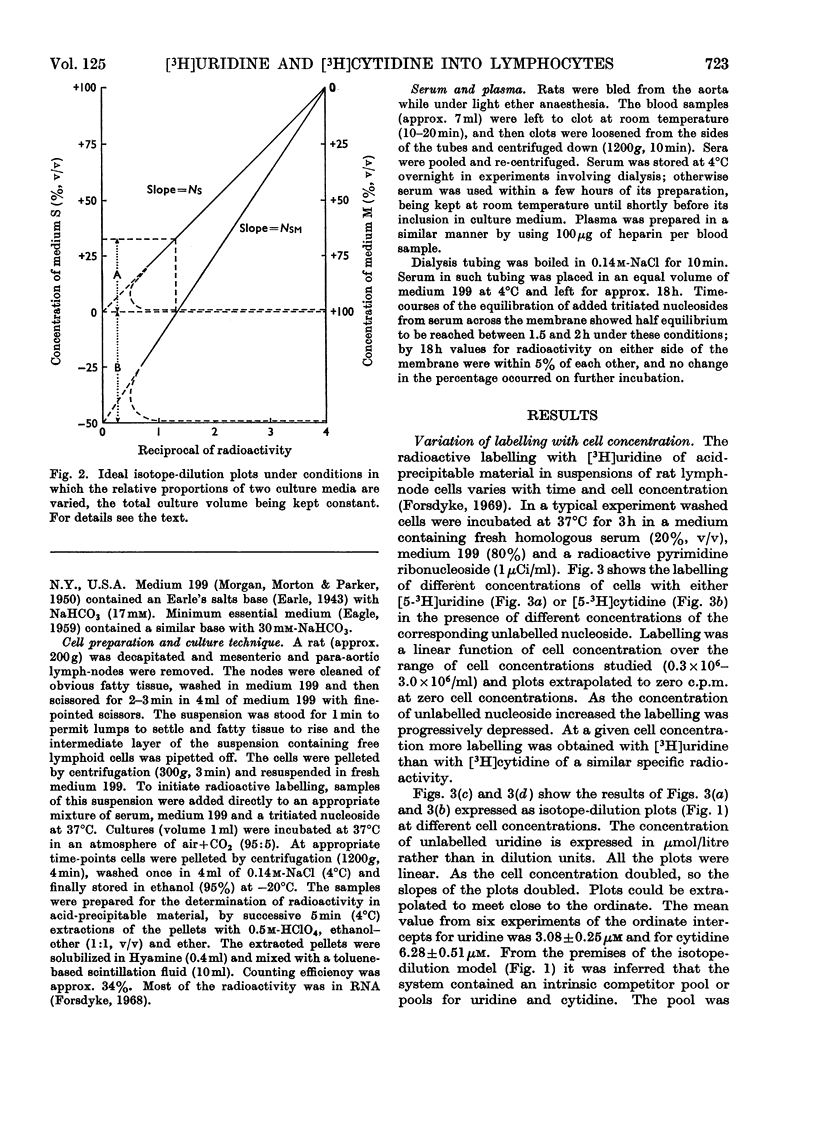
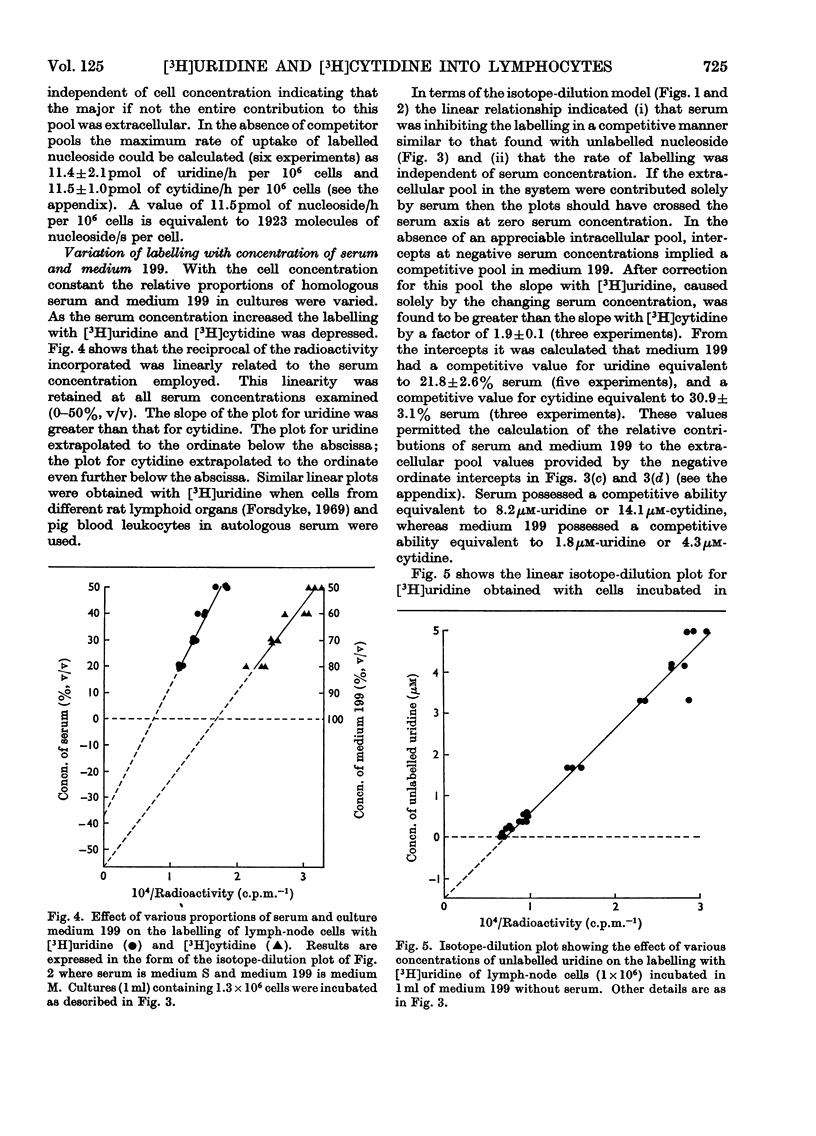
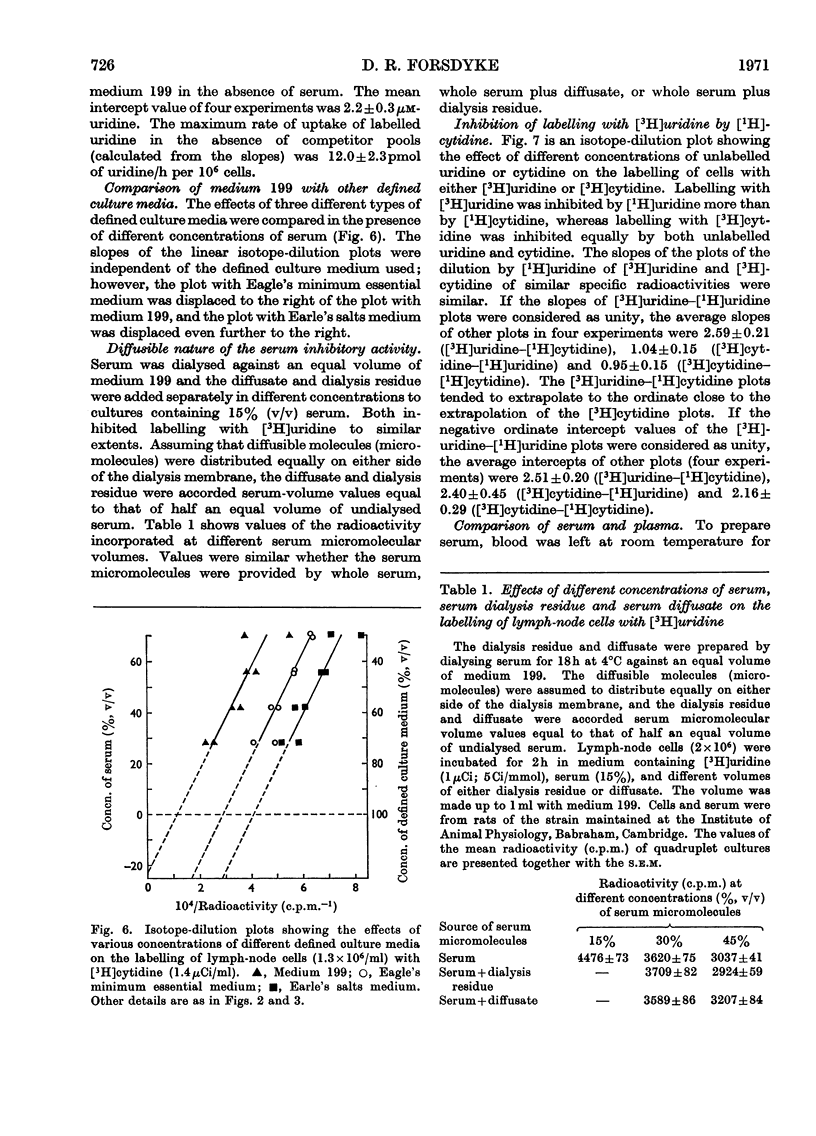
1. Rat lymph-node cells were incubated in serum and medium 199 with [5-3H]uridine or [5-3H]cytidine and acid-precipitable radioactivity was measured. Results were interpreted in terms of an isotope-dilution model. 2. Both serum and medium 199 contained pools that inhibited radioactive labelling in a competitive manner. The serum activity was diffusible and inhibited labelling with [3H]cytidine more than with [3H]uridine; in these respects the activity resembled cytidine (14μm). 3. The pools in serum and plasma were the same size; however, the rate of labelling was greater in plasma, owing to a diffusible factor. 4. Paradoxically, relatively simple media (Earle's salts and Eagle's minimum essential) appeared to have a larger pool than the more complex pyrimidine-containing medium 199; this suggests a contribution to the pool by cells in the simple media. 5. In the absence of pools the average cell was capable of incorporating 2000 radioactive nucleoside molecules/s.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becroft D. M., Phillips L. I., Simmonds A. Hereditary orotic aciduria: long-term therapy with uridine and a trial of uracil. J Pediatr. 1969 Nov;75(5):885–891. doi: 10.1016/s0022-3476(69)80318-5. [DOI] [PubMed] [Google Scholar]
- Brooker G., Appleman M. M. The theoretical basis for the measurement. Biochemistry. 1968 Dec;7(12):4182–4184. doi: 10.1021/bi00852a007. [DOI] [PubMed] [Google Scholar]
- Caldwell I. C., Chan M. F. Tumor cell metabolism in vitro: a study of incubation media. Can J Biochem. 1970 Apr;48(4):517–519. doi: 10.1139/o70-083. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FRIEDKIN M., WOOD H. I. V. Utilization of thymidine-C14 by bone marrow cells and isolated thymus nuclei. J Biol Chem. 1956 Jun;220(2):639–651. [PubMed] [Google Scholar]
- Forsdyke D. R. Impaired activation of thymus lymphocytes by phytohemagglutinin. J Immunol. 1969 Oct;103(4):818–823. [PubMed] [Google Scholar]
- Forsdyke D. R. Quantitiative nucleic acid changes during phytohaemagglutinin-induced lymphocyte transformation in vitro. Dependence of the response on phytohaemagglutinin-serum rati. Biochem J. 1967 Nov;105(2):679–684. doi: 10.1042/bj1050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Quantitiative nucleic acid changes during phytohaemagglutinin-induced lymphocyte transformation in vitro. Dependence of the response on phytohaemagglutinin-serum rati. Biochem J. 1967 Nov;105(2):679–684. doi: 10.1042/bj1050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Studies on the incorporation of [5-3H] uridine during activation and transformation of lymphocytes induced by phytohaemagglutinin. Dependence on the incorporation rate on uridine concentration at certain critical concentrations. Biochem J. 1968 Mar;107(2):197–205. doi: 10.1042/bj1070197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Studies on the incorporation of [5-3H] uridine during activation and transformation of lymphocytes induced by phytohaemagglutinin. Dependence on the incorporation rate on uridine concentration at certain critical concentrations. Biochem J. 1968 Mar;107(2):197–205. doi: 10.1042/bj1070197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander J. E. Limitations of the enzymic-isotope dilution method for the assay of metabolites. Biochim Biophys Acta. 1970 Feb 24;201(2):179–184. doi: 10.1016/0304-4165(70)90291-6. [DOI] [PubMed] [Google Scholar]
- Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5'-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969 Dec;11(2):249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Guir C. D., Swingle K. F., Cole L. J. Plasma deoxycytidine: increased levels after x-irradiation. Proc Soc Exp Biol Med. 1968 Oct;129(1):31–34. doi: 10.3181/00379727-129-33243. [DOI] [PubMed] [Google Scholar]
- Ito K., Uchino H. Control of pyrimidine biosynthesis in human lymphocytes. Induction of glutamine-utilizing carbamyl phosphate synthetase and operation of orotic acid pathway during blastogenesis. J Biol Chem. 1971 Jun 25;246(12):4060–4065. [PubMed] [Google Scholar]
- Lan S. J., Sallach H. J., Cohen P. P. The enzymatic steps of pyrimidine biosynthesis in the unfertilized frog egg. Biochemistry. 1969 Sep;8(9):3673–3680. doi: 10.1021/bi00837a027. [DOI] [PubMed] [Google Scholar]
- Leloir L. F. Nucleoside diphosphate sugars and saccharide synthesis. The fourth Hopkins Memorial Lecture. Biochem J. 1964 Apr;91(1):1.b2–1.b8. [PMC free article] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Roth M. F. Permeation as the rate-limiting step in the phosphorylation of uridine and choline and their incorporation into macromolecules by Novikoff hepatoma cells. Competitive inhibition by phenethyl alcohol, persantin, and adenosine. Biochemistry. 1969 Dec;8(12):4782–4789. doi: 10.1021/bi00840a020. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Nakata Y., Bader J. P. The uptake of nucleosides by cells in culture. I. Inhibition by heterologous nucleosides. Biochim Biophys Acta. 1969 Oct 22;190(2):237–249. doi: 10.1016/0005-2787(69)90075-6. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Ito K. Control of pyrimidine biosynthesis in mammalian tissues. I. Partial purification and characterization of glutamine-utilizing carbamyl phosphate synthetase of mouse spleen and its tissue distribution. J Biol Chem. 1969 Oct 10;244(19):5403–5413. [PubMed] [Google Scholar]


