Abstract
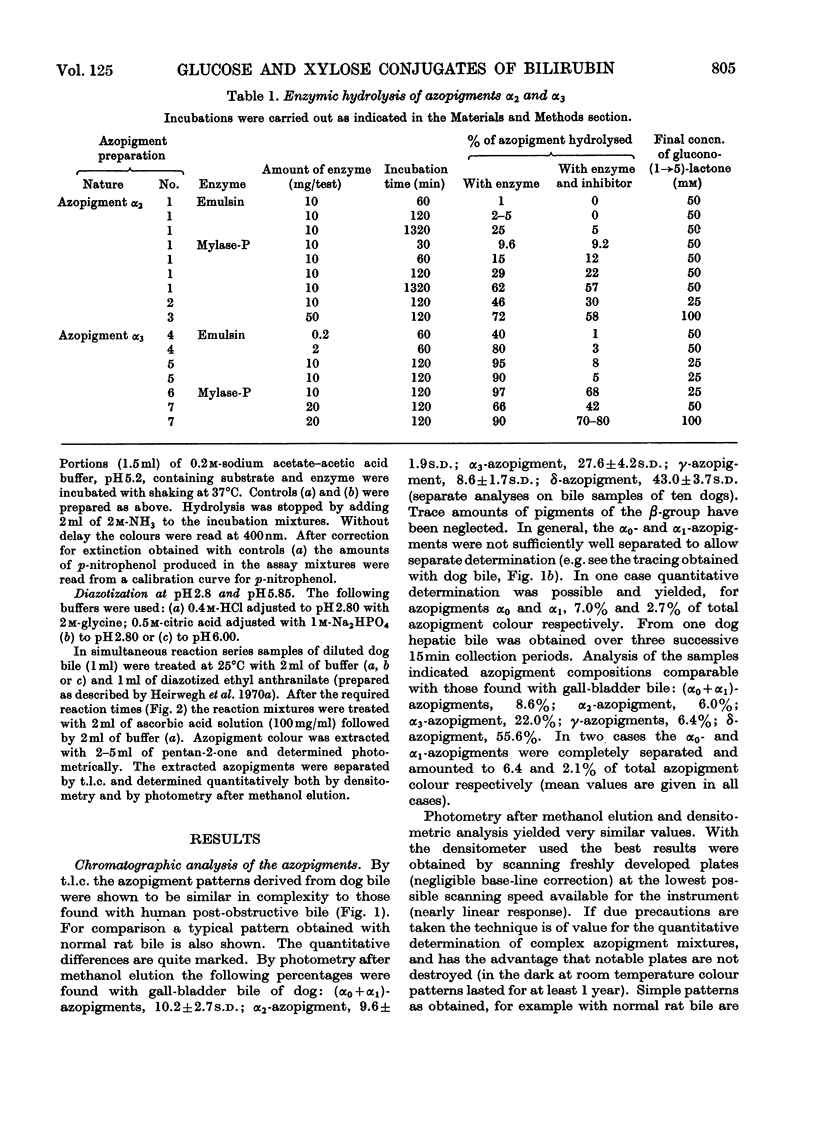
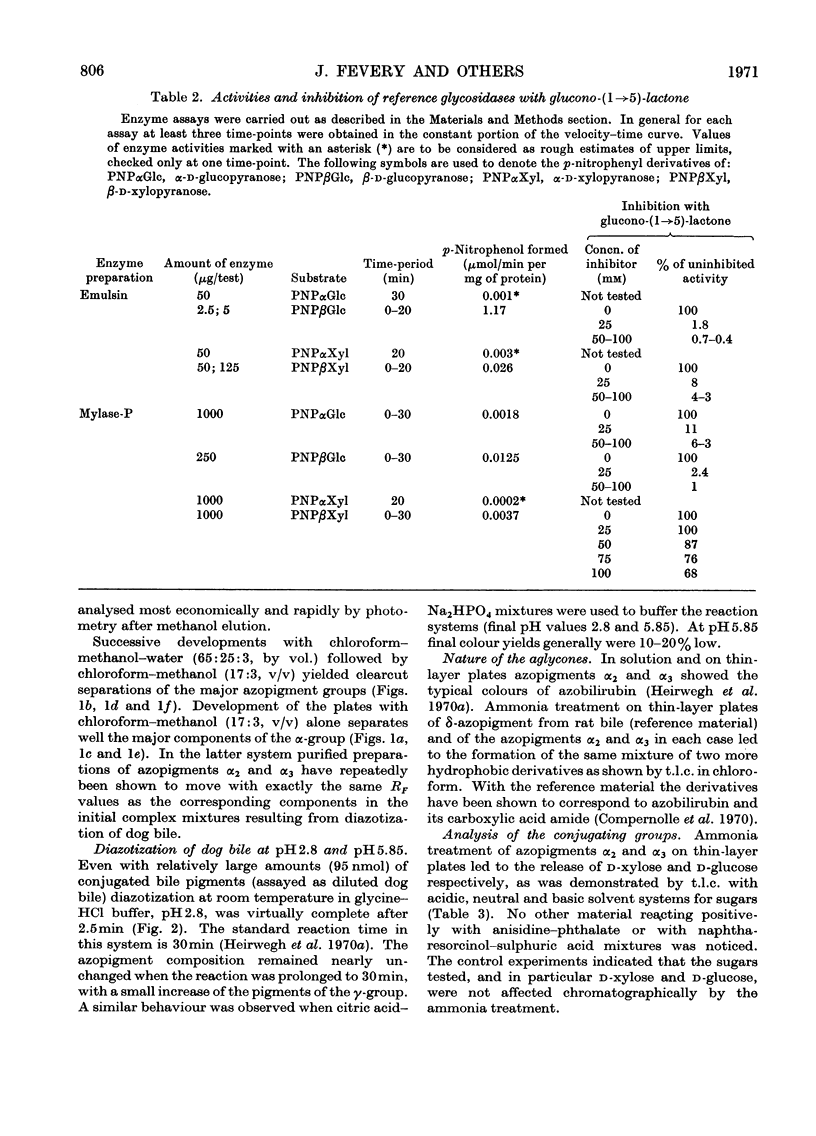
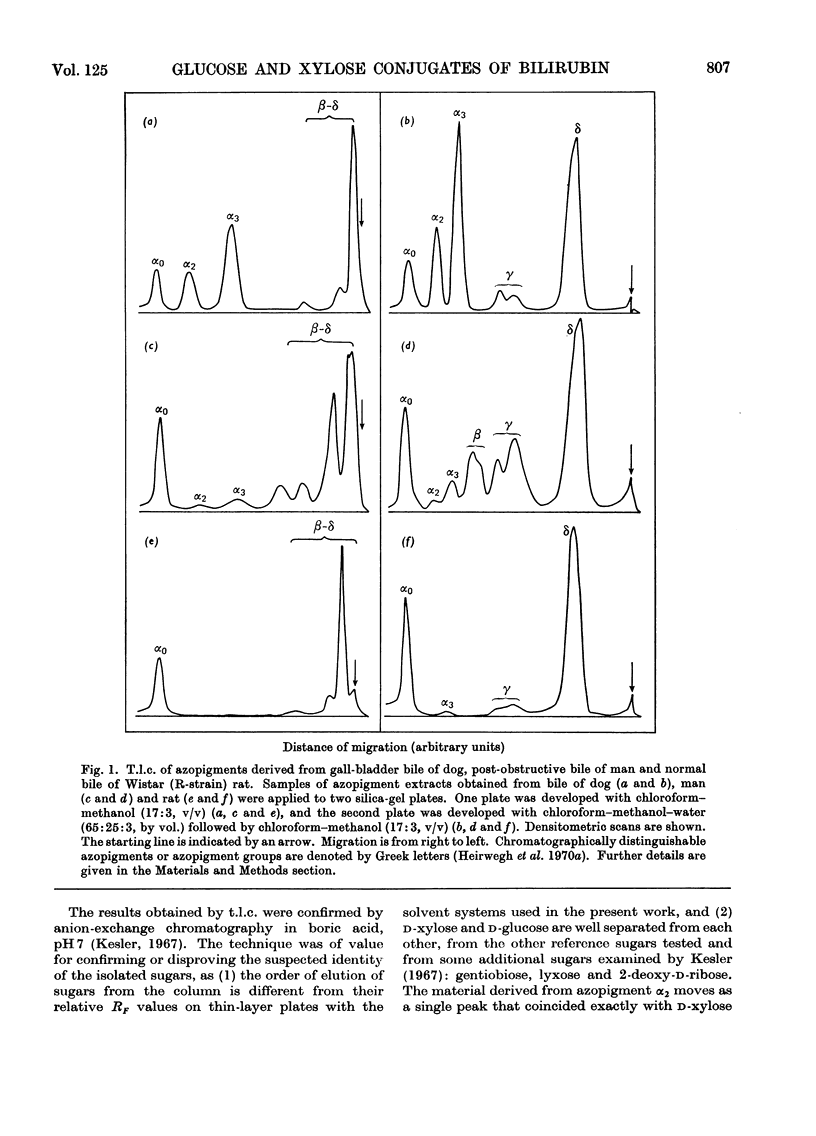
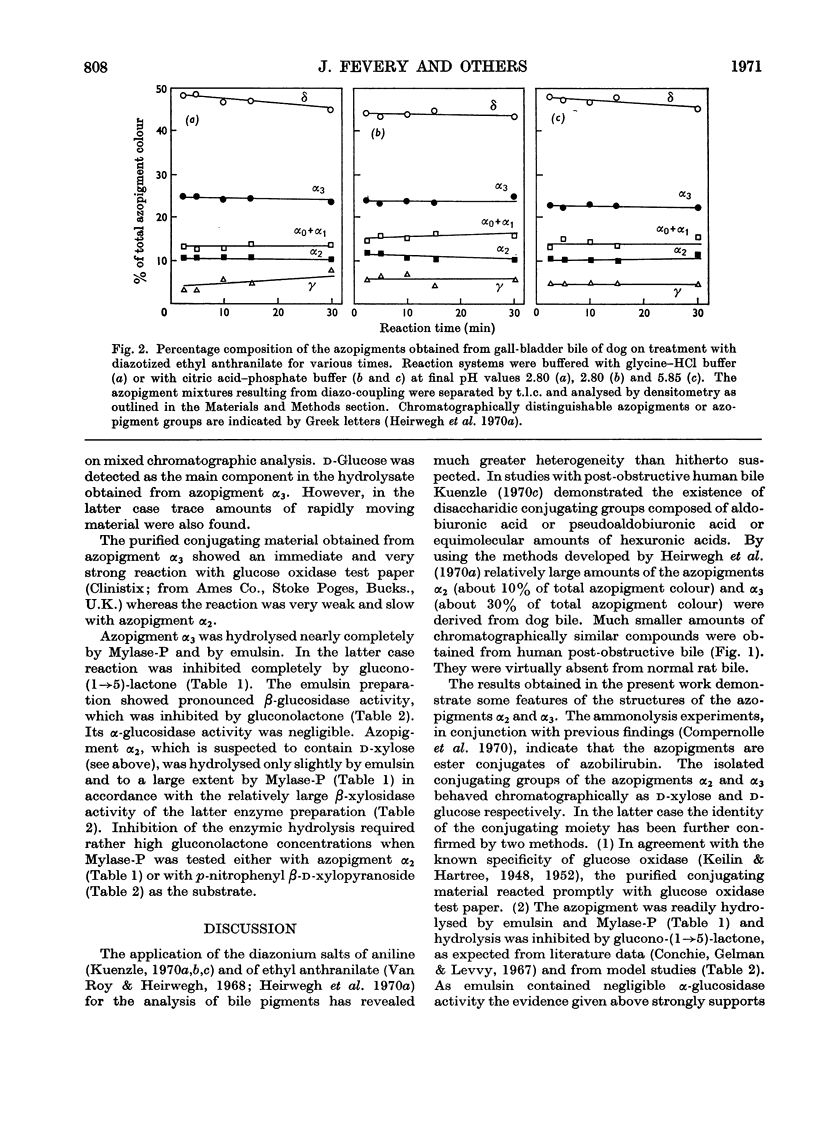
1. T.l.c. with neutral solvent systems of ethyl anthranilate azopigments derived from bile of man, dog and rat revealed pronounced species variation. The less polar components (α-group) could be separated conveniently by development with chloroform–methanol (17:3, v/v). 2. The azopigment material derived from gallbladder bile of dog contained about 10% of azobilirubin β-d-monoxyloside (azopigment α2) and 30% of azobilirubin β-d-monoglucoside (azopigment α3). The sugar moieties were identified by t.l.c. with acidic, neutral and basic solvent systems and by anion-exchange column chromatography of their boric acid complexes. Treatment of the purified azopigments with ammonia vapour led to the formation of the amide of azobilirubin, indicating that both pigments are ester glycosides. The β-d configuration was demonstrated by enzymic studies with emulsin (an adequate source of β-glucosidase activity) and with Mylase-P (an adequate source of β-glucosidase and β-xylosidase activities). 3. Hydrolysis studies with model substrates and with the α2- and α3-azopigments suggested that in Mylase-P the β-glucosidase and β-xylosidase activities reside in separate enzymes. 4. Compared with the accepted conjugation with glucuronic acid as a major route of detoxication in mammals, the detection of large amounts of xylose and glucose conjugates of bilirubin in dog bile suggests that the underlying biosynthetic pathways may be important alternative routes of detoxication.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcos M., Lieberman S. 5-Pregnene-3-beta,20-alpha-diol-3-sulfate-20-(2'-acetamido-2'-deoxy-alpha-D-glucoside) and 5-pregnene-3-beta,20-alpha-diol-3,20-disulfate. Two novel urinary conjugates. Biochemistry. 1967 Jul;6(7):2032–2039. doi: 10.1021/bi00859a022. [DOI] [PubMed] [Google Scholar]
- Bdolah A., Feingold D. S. Decarboxylation of uridine diphosphate-D-glucuronic acid by an enzyme preparation from hen oviduct. Biochem Biophys Res Commun. 1965 Dec 21;21(6):543–546. doi: 10.1016/0006-291x(65)90519-x. [DOI] [PubMed] [Google Scholar]
- Beck C., Tappel A. L. Rat-liver lysosomal beta-glucosidase: a membrane enzyme. Biochim Biophys Acta. 1968 Jan 8;151(1):159–164. doi: 10.1016/0005-2744(68)90170-8. [DOI] [PubMed] [Google Scholar]
- Collins D. C., Jirku H., Layne D. S. Steroid N-acetylglucosaminyl transferase. Localization and some properties of the enzyme in rabbit tissues. J Biol Chem. 1968 Jun 10;243(11):2928–2933. [PubMed] [Google Scholar]
- Collins D. C., Layne D. S. The nature of the glycosidic conjugates of estriol epimers in rabbits. Can J Biochem. 1968 Sep;46(9):1089–1092. doi: 10.1139/o68-162. [DOI] [PubMed] [Google Scholar]
- Collins D. C., Williams K. I., Layne D. S. Further studies on the nature of phenolic steroids in the rabbit and their mode of conjugation. Arch Biochem Biophys. 1967 Sep;121(3):609–613. doi: 10.1016/0003-9861(67)90044-6. [DOI] [PubMed] [Google Scholar]
- Collins D. C., Williamson D. G., Layne D. S. Steroid glucosides. Enzymatic synthesis by a partially purified transferase from rabbit liver microsomes. J Biol Chem. 1970 Feb 25;245(4):873–876. [PubMed] [Google Scholar]
- Compernolle F., Jansen F. H., Heirwegh K. P. Mass-spectrometric study of the azopigments obtained from bile pigments with diazotized ethyl anthranilate. Biochem J. 1970 Dec;120(4):891–894. doi: 10.1042/bj1200891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Fevery J., Heirwegh K. P. Mass-spectrometric structure elucidation of dog bile azopigments as the acyl glycosides of glucopyranose and xylopyranose. Biochem J. 1971 Dec;125(3):811–819. doi: 10.1042/bj1250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchie J., Gelman A. L., Levvy G. A. Inhibition of glycosidases by aldonolactones of corresponding configuration. The C-4- and C-6-specificity of beta-glucosidase and beta-galactosidase. Biochem J. 1967 Jun;103(3):609–615. doi: 10.1042/bj1030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY J. D., LAURENT T. C., RODEN L. ENZYMATIC DEGRADATION OF CHONDROMUCOPROTEIN. J Biol Chem. 1964 Oct;239:3312–3320. [PubMed] [Google Scholar]
- Grebner E. E., Hall C. W., Neufeld E. F. Glycosylation of serine residues by a uridine diphosphate-xylose: protein xylosyltransferase from mouse mastocytoma. Arch Biochem Biophys. 1966 Sep 26;116(1):391–398. doi: 10.1016/0003-9861(66)90045-2. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Compernolle F., Fevery J. Excretion of bilirubin conjugated with glucose in dog bile. Biochem J. 1970 Dec;120(4):17P–17P. doi: 10.1042/bj1200017pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Specificity of glucose oxidase (notatin). Biochem J. 1952 Jan;50(3):331–341. doi: 10.1042/bj0500331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of glucose oxidase (notatin): Addendum. Sedimentation and diffusion of glucose oxidase (notatin). Biochem J. 1948;42(2):221–229. [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Isolation of phenylazo derivatives of bile bilirubin. Biochem J. 1970 Sep;119(3):387–394. doi: 10.1042/bj1190387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Nuclear-magnetic-resonance, infrared and optical spectra of model compounds. Biochem J. 1970 Sep;119(3):395–409. doi: 10.1042/bj1190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAYNE D. S. IDENTIFICATION IN RABBIT URINE OF THE 3-GLUCURONOSIDE-17-N-ACETYLGLUCOSAMINIDE OF 17-ALPHA-ESTRADIOL. Endocrinology. 1965 Apr;76:600–603. doi: 10.1210/endo-76-4-600. [DOI] [PubMed] [Google Scholar]
- LAYNE D. S., SHETH N. A., KIRDANI R. Y. THE ISOLATION FROM RABBIT URINE OF A CONJUGATE OF 17-ALPHA-ESTRADIOL WITH N-ACETYLGLUCOSAMINE. J Biol Chem. 1964 Oct;239:3221–3225. [PubMed] [Google Scholar]
- Lindahl U. Glucuronic acid- and glucosamine-containing oligosaccharides from the heparin-protein linkage region. Biochim Biophys Acta. 1968 Feb 1;156(1):203–206. doi: 10.1016/0304-4165(68)90124-4. [DOI] [PubMed] [Google Scholar]
- MARSH C. A. Quercetin glucosiduronic acid from the French bean. Nature. 1955 Jul 23;176(4473):176–177. doi: 10.1038/176176b0. [DOI] [PubMed] [Google Scholar]
- Matsui M., Fukushima D. K. On the configuration of naturally occurring steroid N-acetylglucosaminides. Biochemistry. 1969 Jul;8(7):2997–3000. doi: 10.1021/bi00835a047. [DOI] [PubMed] [Google Scholar]
- SCHAYER R. W. The metabolism of histamine in various species. Br J Pharmacol Chemother. 1956 Dec;11(4):472–473. doi: 10.1111/j.1476-5381.1956.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert J. E., DeLuca S. The synthesis of uridine diphosphate xylose by particulate preparations from mouse mast-cell tumors. Biochim Biophys Acta. 1967 Jun 13;141(1):193–196. doi: 10.1016/0304-4165(67)90263-2. [DOI] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. G., Collins D. C., Layne D. S., Conrow R. B., Bernstein S. Isolation of 17-alpha-estradiol 17-beta-D-glucopyranoside from rabbit urine, and its synthesis and characterization. Biochemistry. 1969 Nov;8(11):4299–4304. doi: 10.1021/bi00839a012. [DOI] [PubMed] [Google Scholar]
- Williamson D. G., Layne D. S. The hydrolysis of estrogen glucosides by various enzyme preparations. Steroids. 1970 Mar;15(3):327–332. doi: 10.1016/s0039-128x(70)80052-6. [DOI] [PubMed] [Google Scholar]


