Abstract
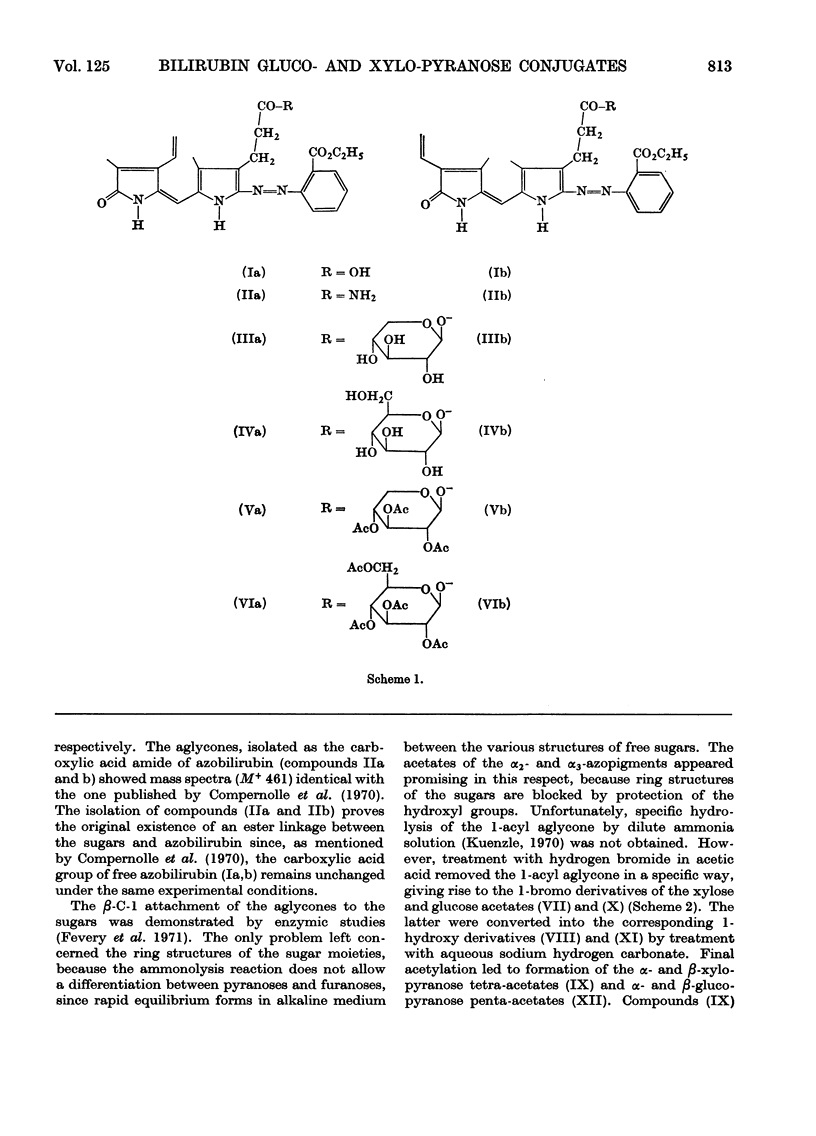
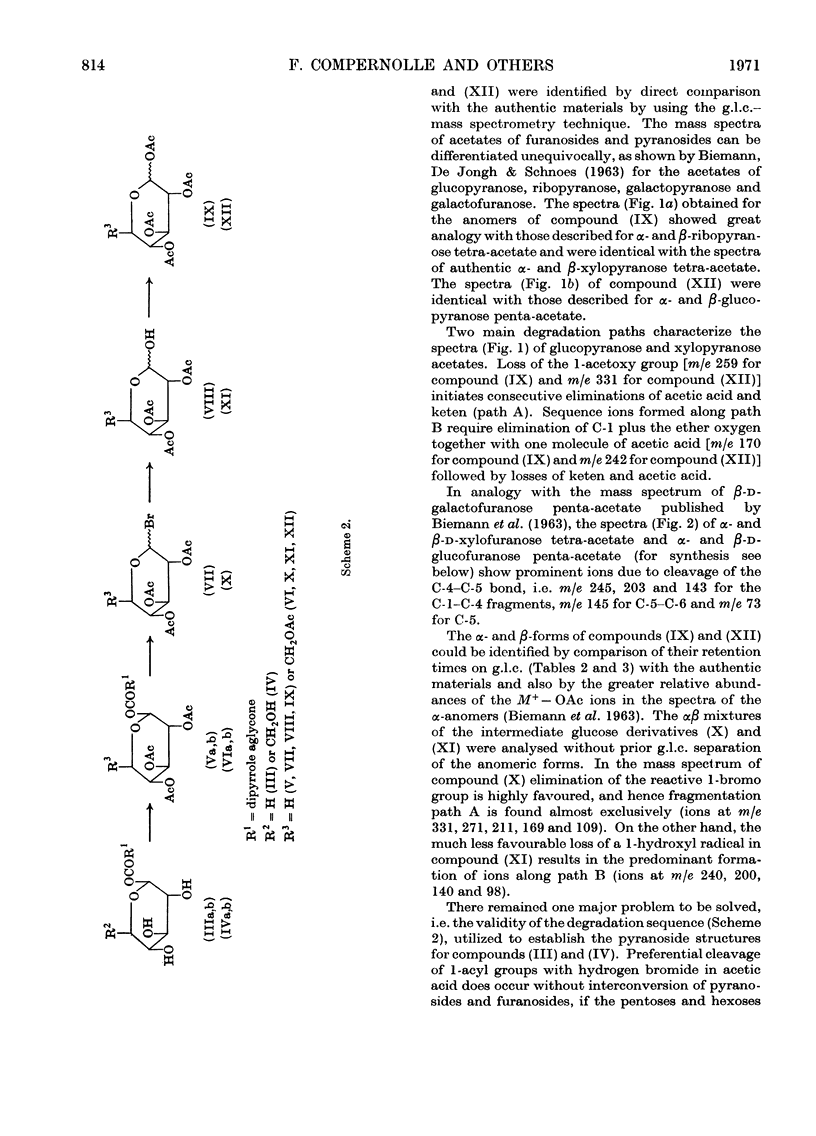
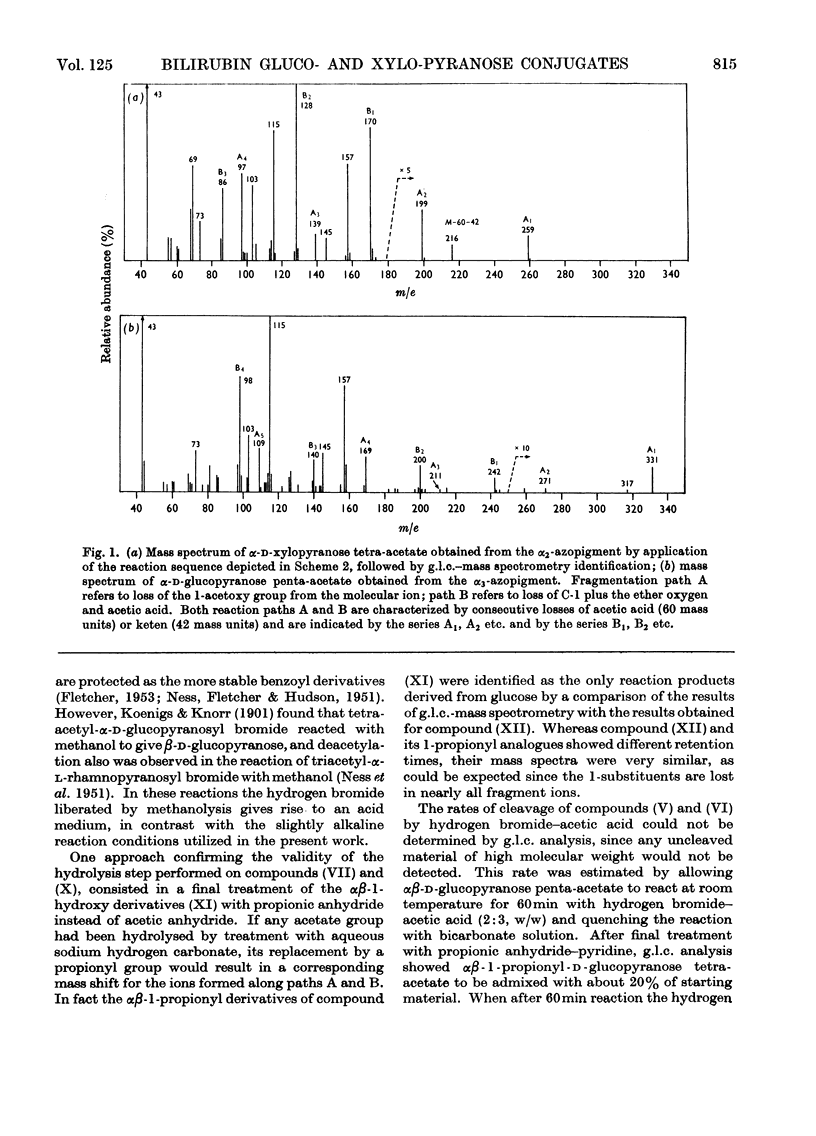
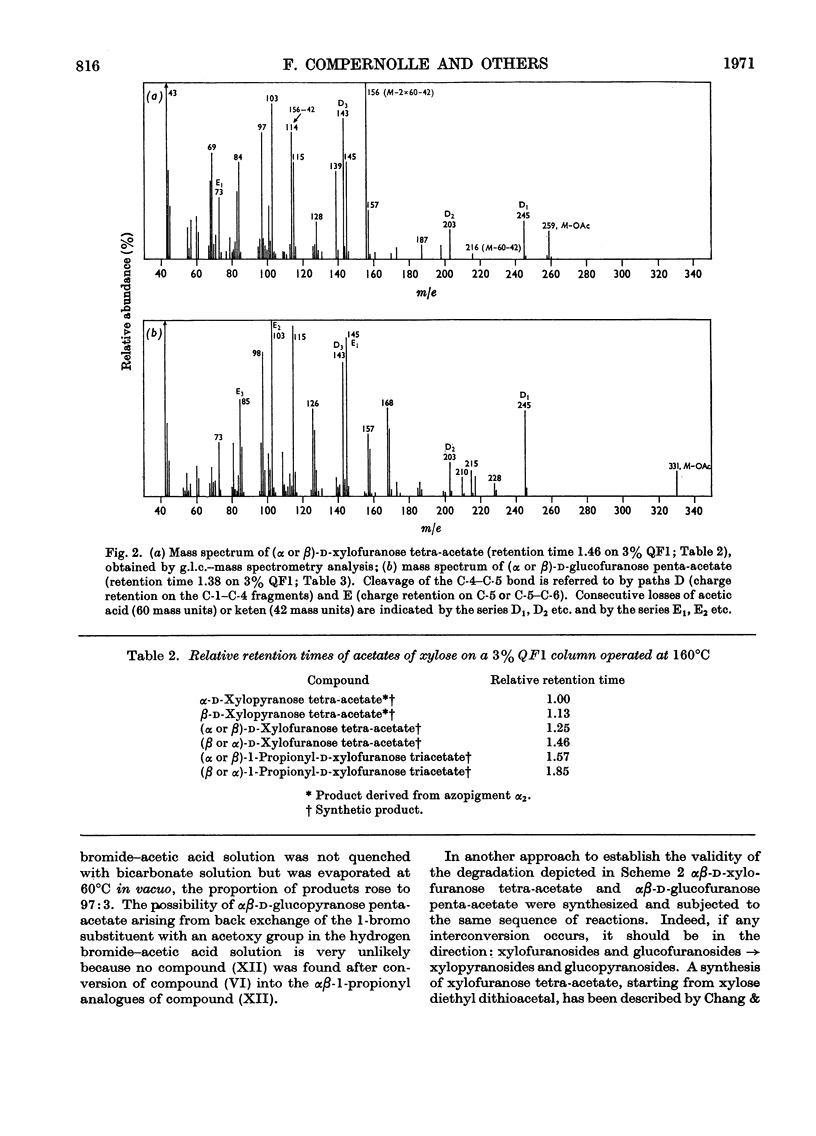
1. The structures of the α2- and α3-azopigments, prepared by diazotization of dog bile with ethyl anthranilate, were shown by mass spectrometry and g.l.c. to correspond to azobilirubin β-d-xylopyranoside and azobilirubin β-d-glucopyranoside respectively. 2. Both azopigments consist of a mixture of two methyl vinyl isomers having structures (IIIa) and (IIIb) for the α2-azopigment and structures (IVa) and (IVb) for the α3-azopigment. Separation of methyl vinyl isomers was obtained by t.l.c. or column chromatography performed on the acetylated azopigments. Hydrolysis of the less polar acetates derived from components (IIIa) and (IVa) gave rise to the azopigment (Ia), whereas hydrolysis of the more polar acetates derived from components (IIIb) and (IVb) gave rise to the azopigment acid (Ib). The positions of methyl and vinyl substituents in compounds (Ia) and (Ib) were assigned on the basis of their n.m.r. spectra. 3. Molecular ions in the mass spectra of the trimethylsilyl and acetyl derivatives of the azopigments indicated the presence of a pentose and a hexose conjugating sugar. 4. The ester functions linking the sugars to the propionic acid side chain of azobilirubin were demonstrated by ammonolysis and identification of the amide of azobilirubin as the aglycone derivative. 5. The sugar moieties were shown to occur as xylopyranose (α2) and glucopyranose (α3), bound at C-1, by application of a sequence of reactions performed on a micro-scale. The sugar hydroxyl groups were acetylated and the 1-acyl aglycone removed selectively by treatment with hydrogen bromide in acetic acid. Hydrolysis of the 1-bromo sugar acetates followed by acetylation afforded the α- and β-xylopyranose tetra-acetates and α- and β-glucopyranose penta-acetates, identified by a combination of g.l.c. and mass spectrometry. 6. The validity of this degradation scheme was confirmed (a) by g.l.c.–mass spectrometry identification of the α- and β-1-propionyl derivatives of glucopyranose tetra-acetate, obtained from the α3-azopigment after final reaction with propionic anhydride; (b) by subjecting the acetates of αβ-glucopyranose, αβ-xylofuranose and αβ-glucofuranose to the same sequence of reactions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Compernolle F., Jansen F. H., Heirwegh K. P. Mass-spectrometric study of the azopigments obtained from bile pigments with diazotized ethyl anthranilate. Biochem J. 1970 Dec;120(4):891–894. doi: 10.1042/bj1200891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]


