Abstract
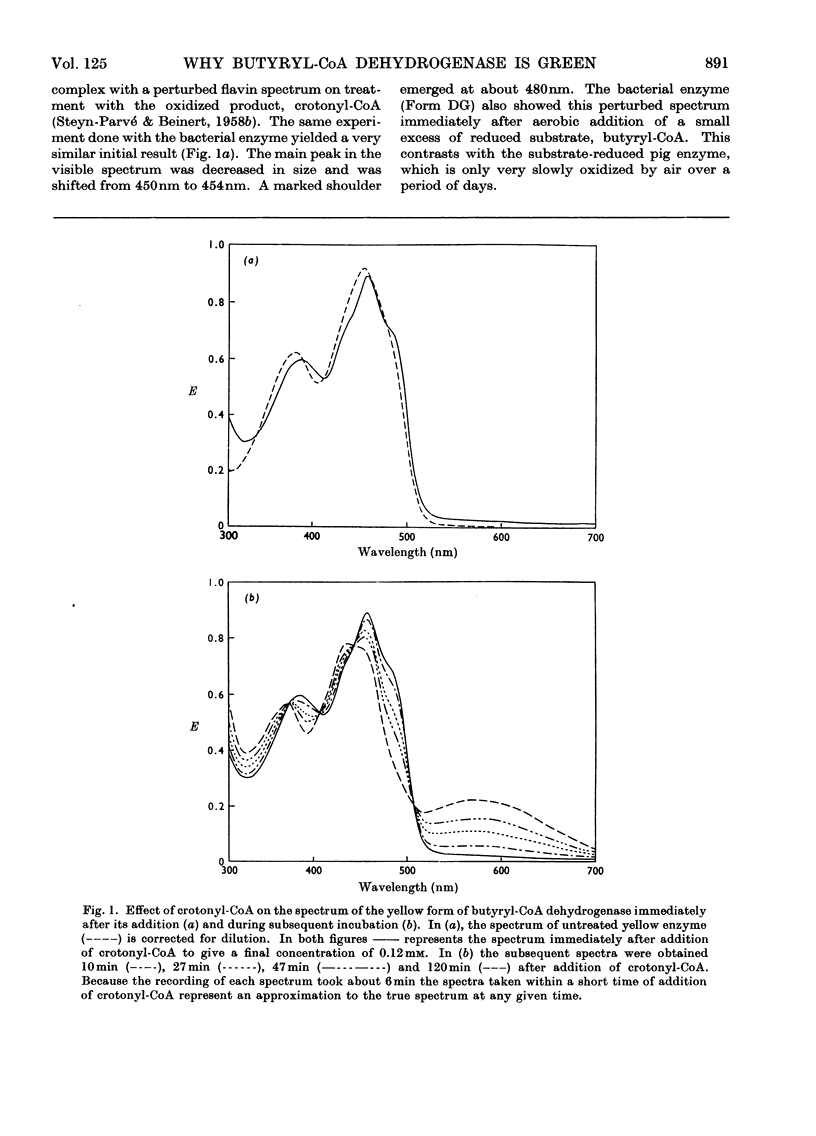
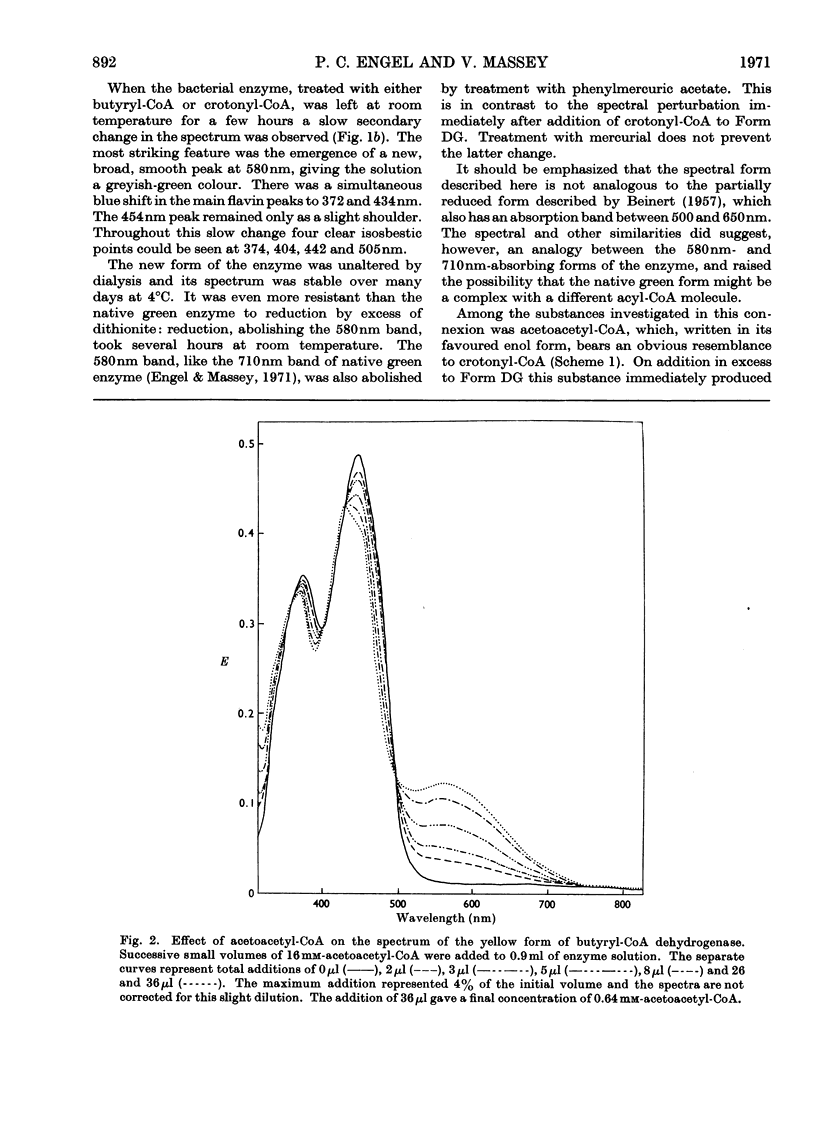
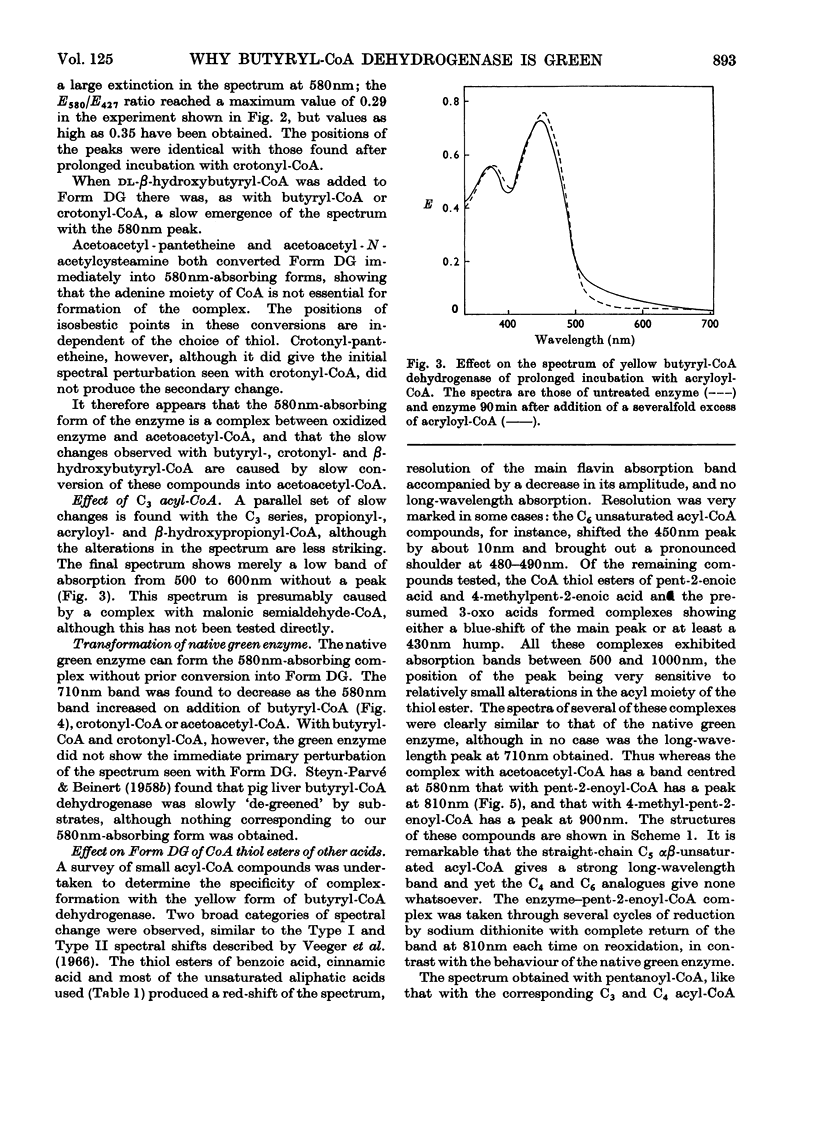
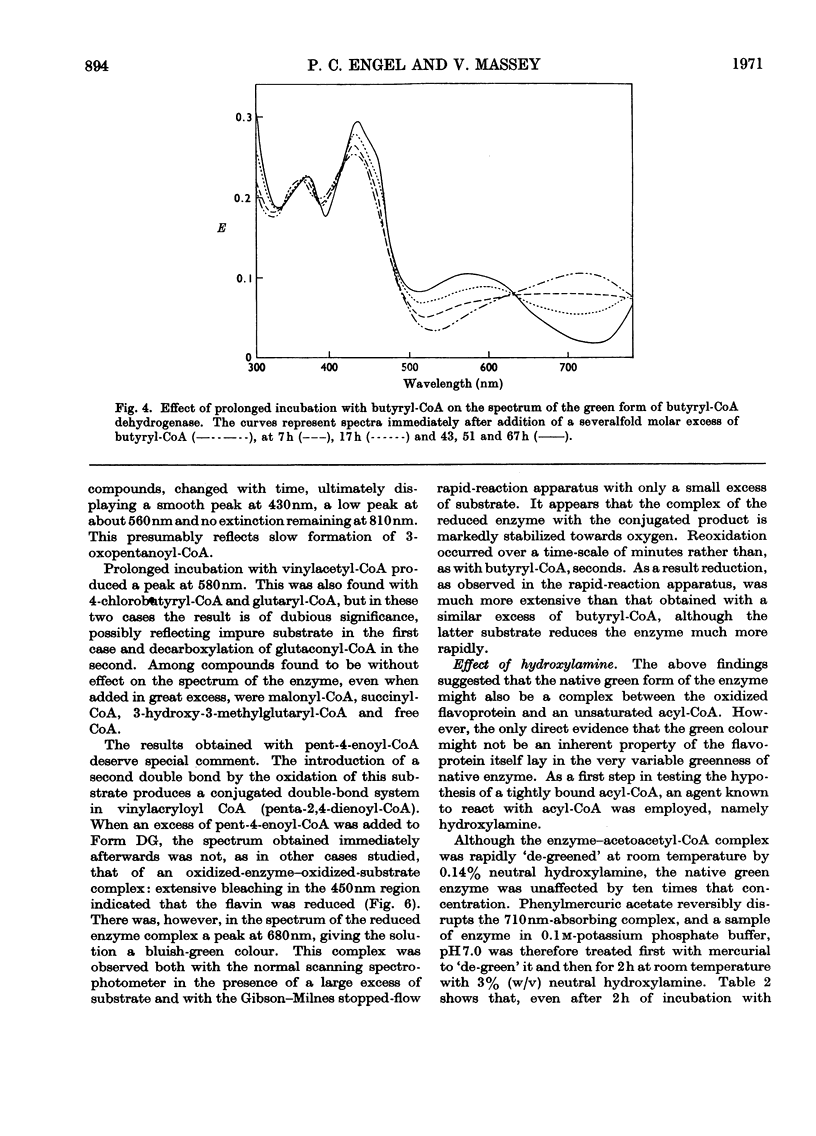
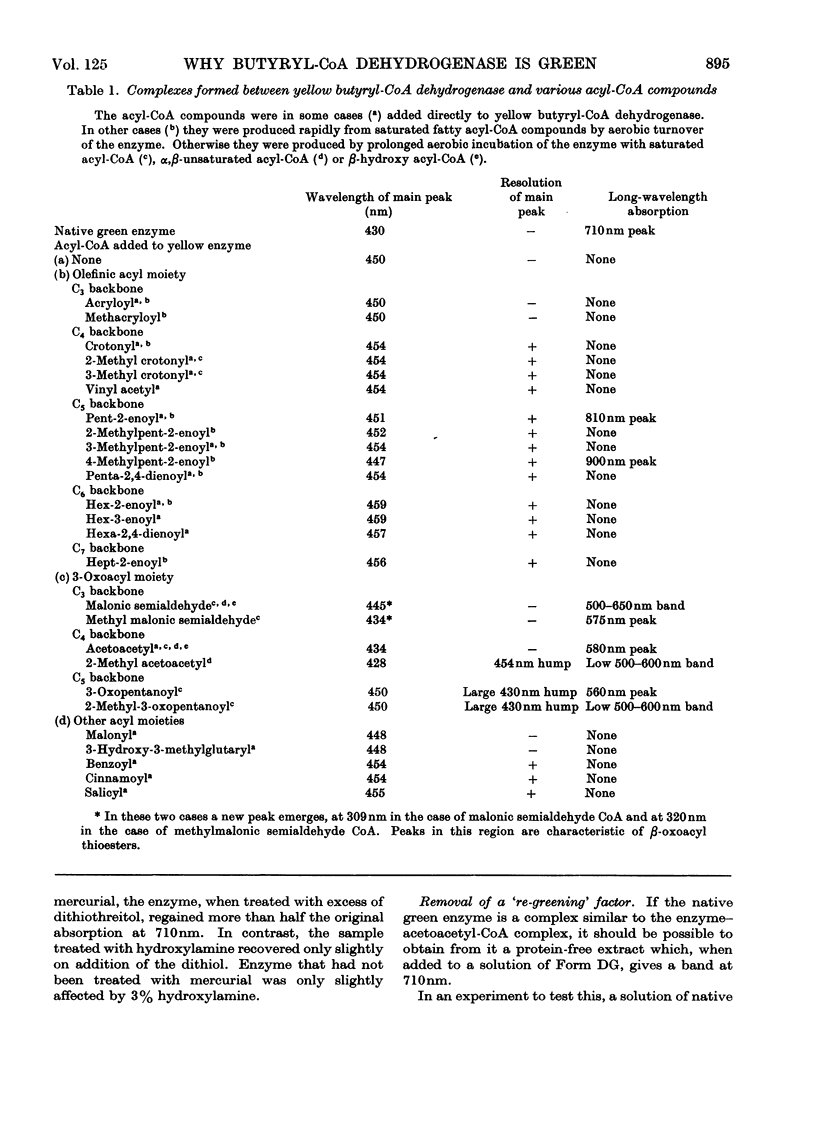
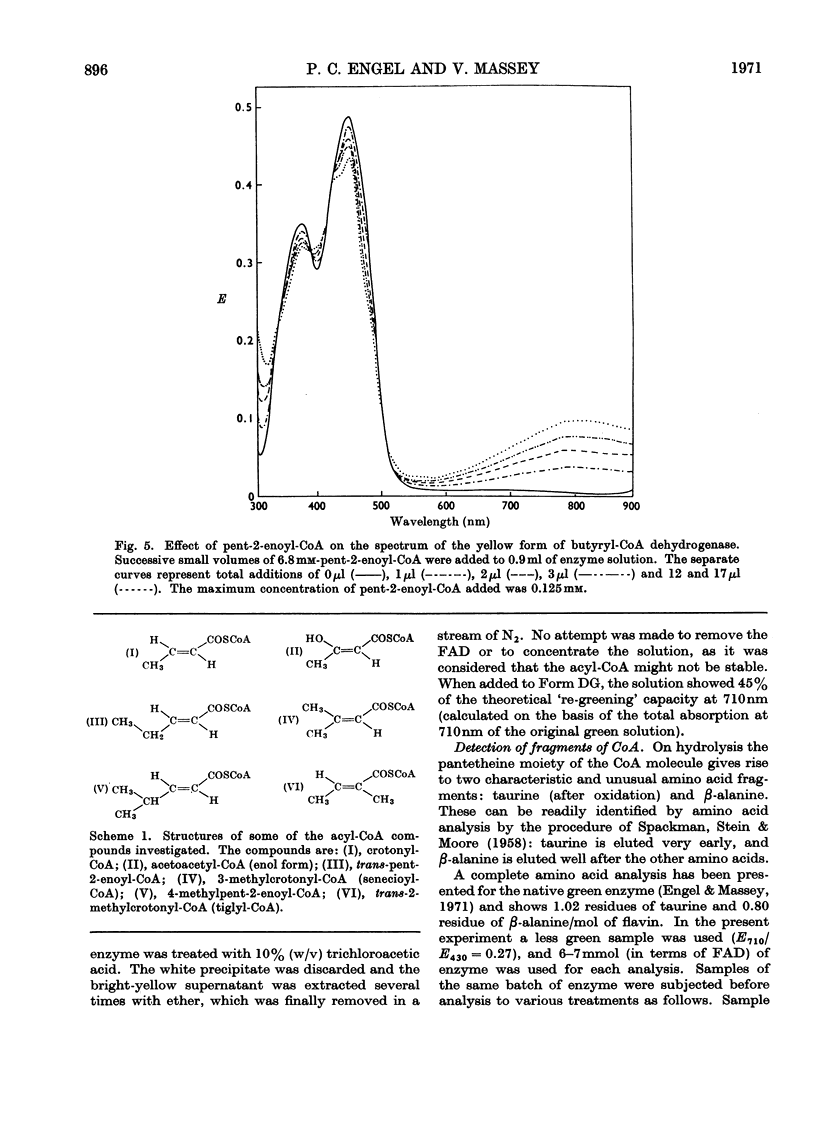
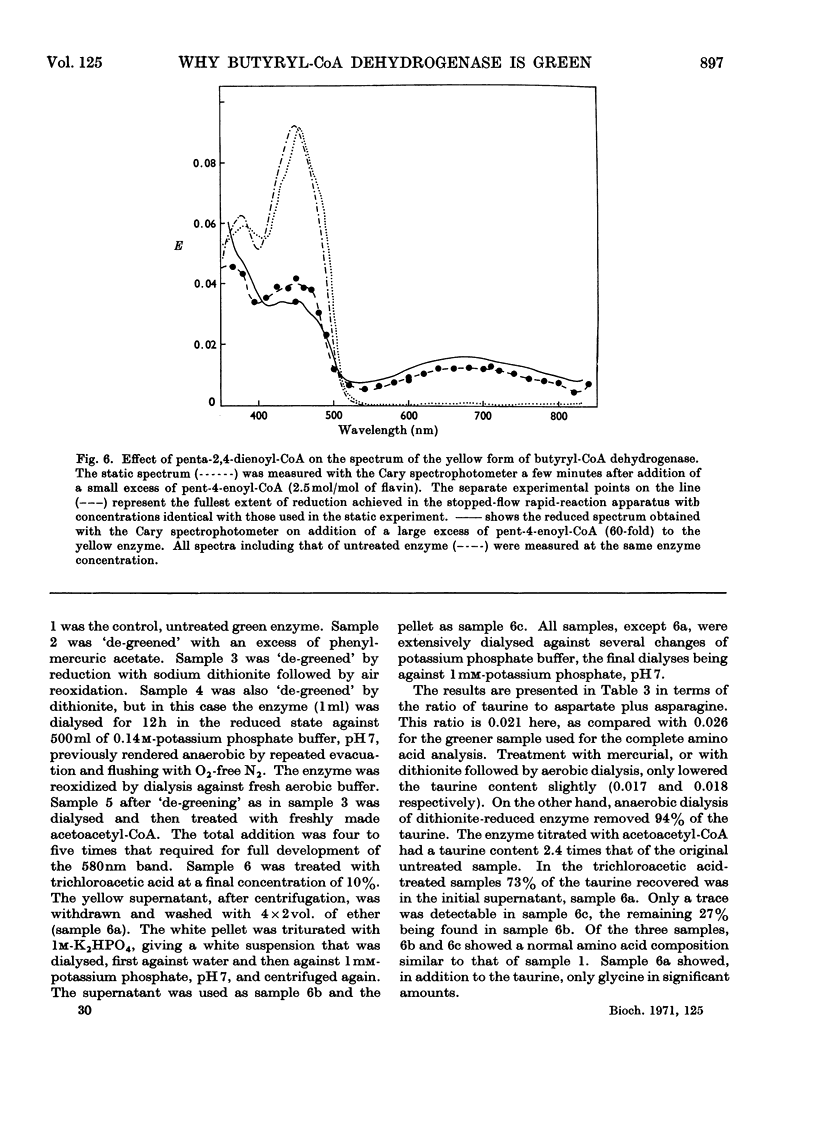
1. Butyryl-CoA dehydrogenase from Peptostreptococcus elsdenii forms very tightly bound complexes with various acyl-CoA compounds. Spectra in some cases merely show resolution of the 450nm band, but those with acetoacetyl-, pent-2-enoyl- and 4-methylpent-2-enoyl-CoA show long-wavelength bands similar to the 710nm band of native enzyme. These complexes are formed instantaneously by the yellow form of the enzyme and much more slowly by the green form. 2. An acid extract of the green enzyme reconverts the yellow into the green form. 3. Hydroxylamine makes irreversible the otherwise reversible conversion of the green enzyme into the yellow form by phenylmercuric acetate. 4. Amino acid analysis for taurine and β-alanine shows approx. 1mol of CoA/mol of flavin in green enzyme. Anaerobic dialysis of reduced enzyme removes the CoA. On acid precipitation of green enzyme the CoA is found only in the supernatant. 5. It is concluded that native green enzyme is probably complexed with unsaturated acyl-CoA. This is shown to be consistent with findings of other workers. Catalytic activity requires displacement of the acyl-CoA, which is therefore likely to be a potent inhibitor. 6. An explanation is offered for the irreversible conversion of green into yellow enzyme by sodium dithionite. 7. The enzyme displays a feeble, previously undetected, activity towards β-hydroxybutyryl-CoA. 8. The product of oxidation of pent-4-enoyl-CoA forms a complex with reduced enzyme and strongly inhibits reoxidation of the FAD. This may contribute to inhibition of fatty acid oxidation by pent-4-enoic acid in mammals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H. Evidence for an intermediate in the oxidation-reduction of flavoproteins. J Biol Chem. 1957 Mar;225(1):465–478. [PubMed] [Google Scholar]
- BROWN G. M., SNELL E. E. The relationship of pantethine to naturally occurring forms of the Lactobacillus bulgaricus factor. J Biol Chem. 1952 Sep;198(1):375–383. [PubMed] [Google Scholar]
- Bressler R., Corredor C., Brendel K. Hypoglycin and hypoglycin-like compounds. Pharmacol Rev. 1969 Jun;21(2):105–130. [PubMed] [Google Scholar]
- CRANE F. L., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. II. The electron-transferring flavoprotein. J Biol Chem. 1956 Feb;218(2):717–731. [PubMed] [Google Scholar]
- Cornforth J. W., Cornforth R. H., Popják G., Yengoyan L. Studies on the biosynthesis of cholesterol. XX. Steric course of decarboxylation of 5-pyrophosphomevalonate and of the carbon to carbon bond formation in the biosynthesis of farnesyl pyrophosphate. J Biol Chem. 1966 Sep 10;241(17):3970–3987. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Massey V. The purification and properties of butyryl-coenzyme A dehydrogenase from Peptostreptococcus elsdenii. Biochem J. 1971 Dec;125(3):879–887. doi: 10.1042/bj1250879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRISELL W. R., HELLERMAN L., LOWE H. J. Flavoenzyme catalysis: substrate-competitive inhibition of D-amino acid oxidase. J Biol Chem. 1956 Nov;223(1):75–83. [PubMed] [Google Scholar]
- GREEN D. E., MII S., MAHLER H. R., BOCK R. M. Studies on the fatty acid oxidizing system of animal tissues. III. Butyryl coenzyme A dehydrogenase. J Biol Chem. 1954 Jan;206(1):1–12. [PubMed] [Google Scholar]
- Hoskins D. D. The electron-transferring flavoprotein as a common intermediate in the mitochondrial oxidation of butyryl coenzyme A and sarcosine. J Biol Chem. 1966 Oct 10;241(19):4472–4479. [PubMed] [Google Scholar]
- MAHLER H. R. Studies on the fatty acid oxidizing system of animal tissues. IV. The prosthetic group of butyryl coenzyme A dehydrogenase. J Biol Chem. 1954 Jan;206(1):13–26. [PubMed] [Google Scholar]
- MASSEY V., GIBSON Q. H. ROLE OF SEMIQUINONES IN FLAVOPROTEIN CATALYSIS. Fed Proc. 1964 Jan-Feb;23:18–29. [PubMed] [Google Scholar]
- MASSEY V., PALMER G. Charge transfer complexes of lipoyl dehydrogenase and free flavins. J Biol Chem. 1962 Jul;237:2347–2358. [PubMed] [Google Scholar]
- Massey V., Curti B., Müller F., Mayhew S. G. On the reaction of borohydride with D- and L-amino acid oxidases. J Biol Chem. 1968 Mar 25;243(6):1329–1330. [PubMed] [Google Scholar]
- Massey V., Ganther H. On the interpretation of the absorption spectra of flavoproteins with special reference to D-amino acid oxidase. Biochemistry. 1965 Jun;4(6):1161–1173. doi: 10.1021/bi00882a027. [DOI] [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Matthews R. G., Massey V. Isolation of old yellow enzyme in free and complexed forms. J Biol Chem. 1969 Apr 10;244(7):1779–1786. [PubMed] [Google Scholar]
- STEYN-PARVE E. P., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. VI. Isolation and properties of stable enzyme-substrate complexes. J Biol Chem. 1958 Oct;233(4):843–852. [PubMed] [Google Scholar]
- STEYN-PARVE E. P., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. VII. The nature of the green color of butyryl dehydrogenase. J Biol Chem. 1958 Oct;233(4):853–861. [PubMed] [Google Scholar]
- Sherratt H. S. Hypoglycin and related hypoglycaemic compounds. Br Med Bull. 1969 Sep;25(3):250–255. doi: 10.1093/oxfordjournals.bmb.a070713. [DOI] [PubMed] [Google Scholar]
- Tishler S. L., Goldman P. Properties and reactions of salicyl-coenzyme A. Biochem Pharmacol. 1970 Jan;19(1):143–150. doi: 10.1016/0006-2952(70)90335-7. [DOI] [PubMed] [Google Scholar]
- VAGELOS P. R., EARL J. M. Propionic acid metabolism. III. beta-Hydroxypropionyl coenzyme A and malonyl semialdehyde coenzyme A, intermediates in propionate oxidation by Clostridium kluyveri. J Biol Chem. 1959 Sep;234:2272–2280. [PubMed] [Google Scholar]
- YAGI K., OZAWA T. Complex formation of apo-enzyme, coenzyme and substrate of D-amino acid oxidase. II. Spectrophotometric analysis using a substrate-substitute. Biochim Biophys Acta. 1962 Jan 29;56:413–419. doi: 10.1016/0006-3002(62)90592-9. [DOI] [PubMed] [Google Scholar]


