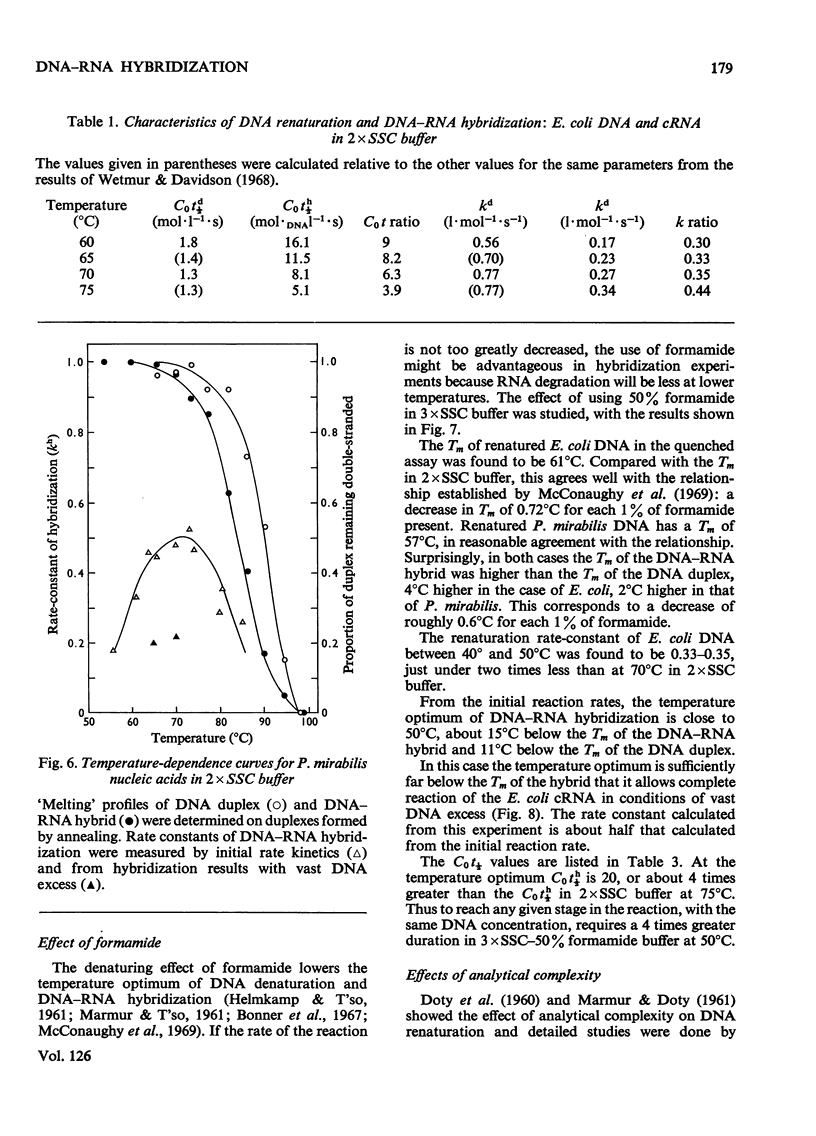
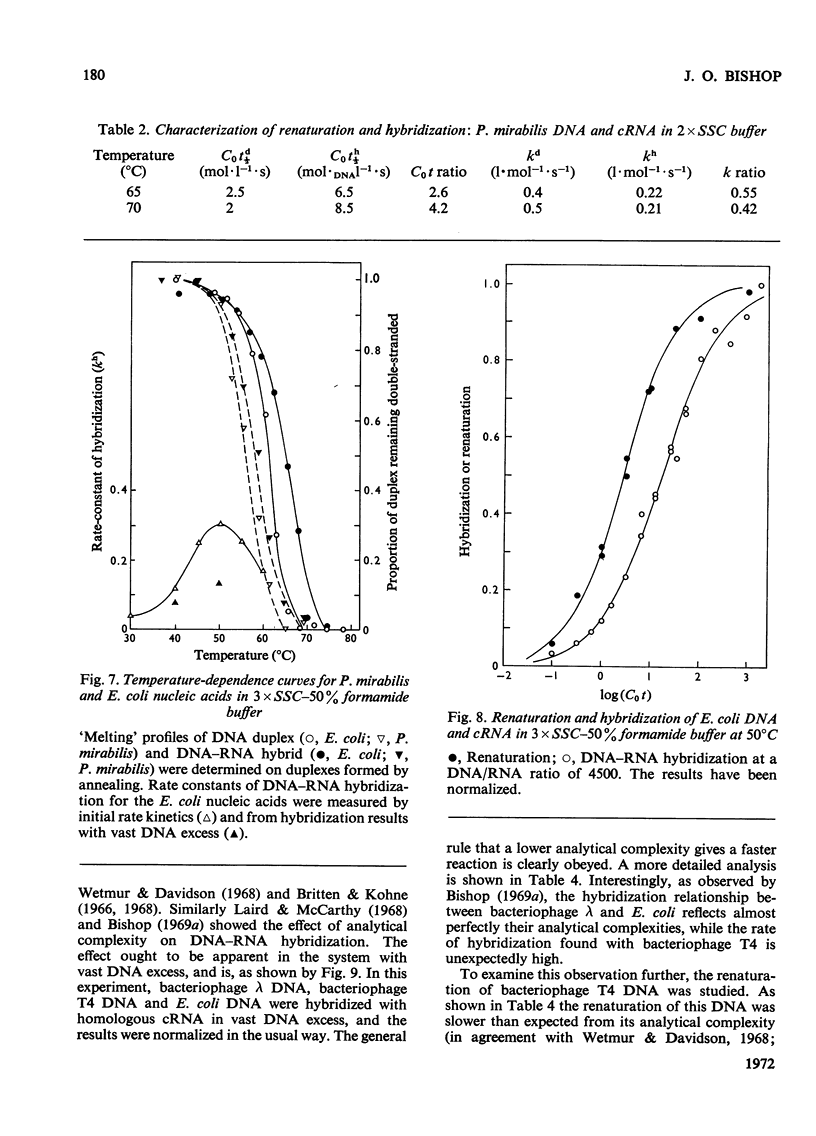
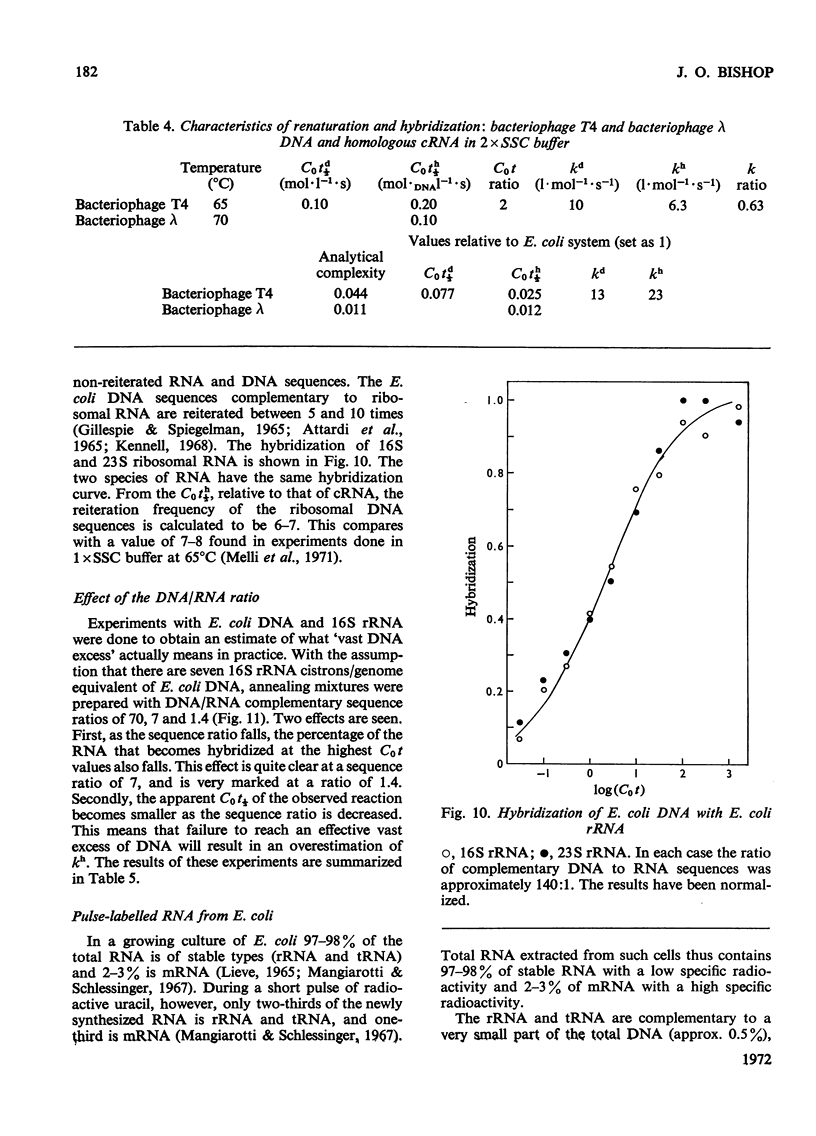
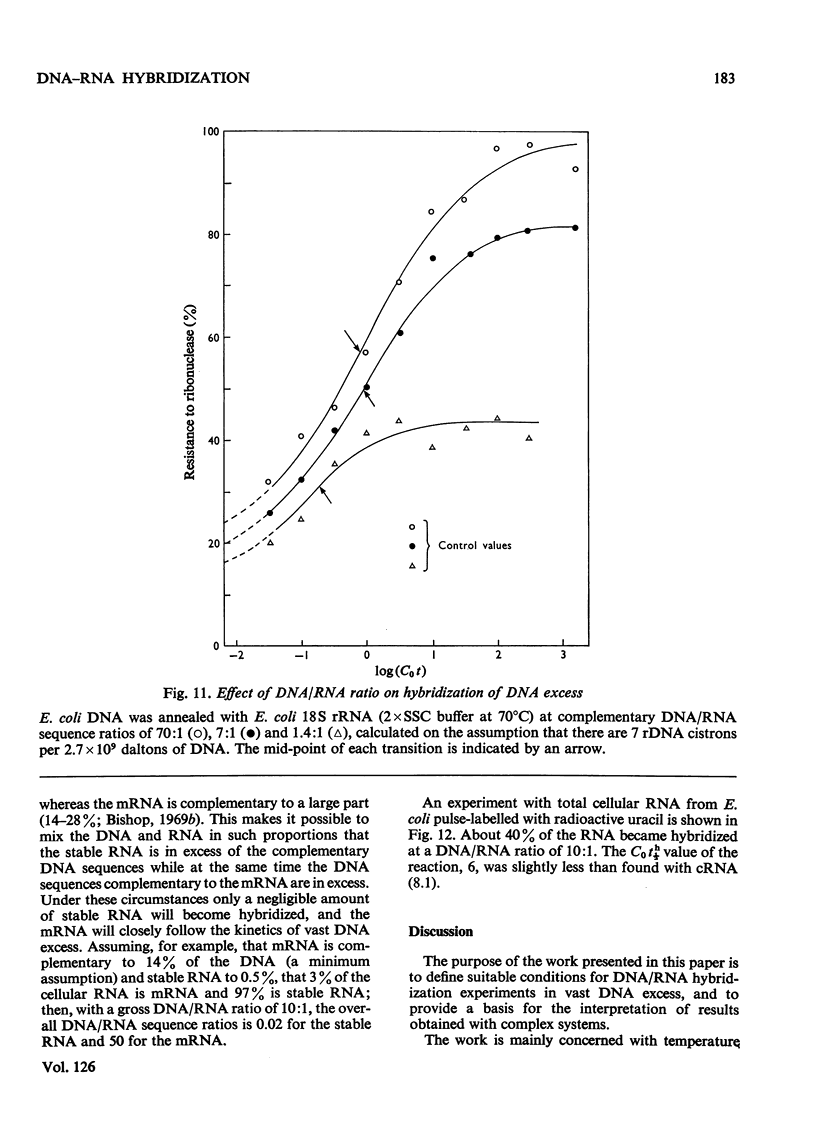
Abstract
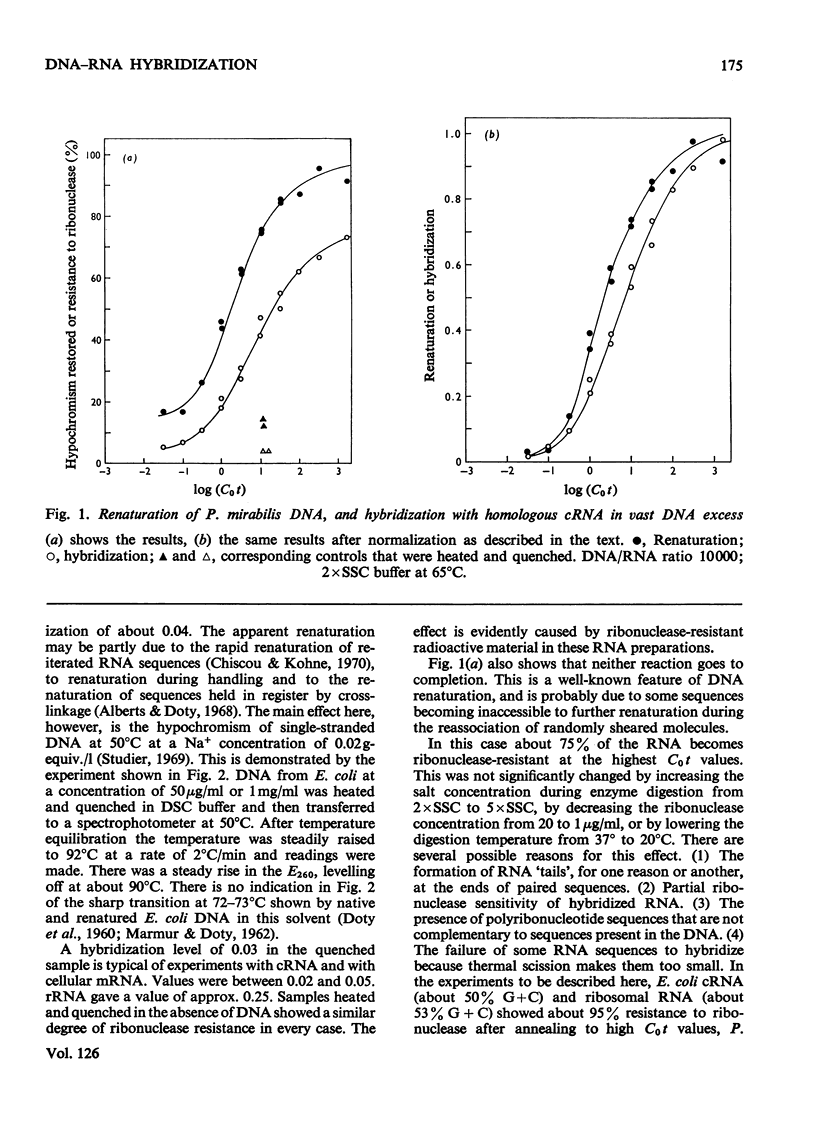
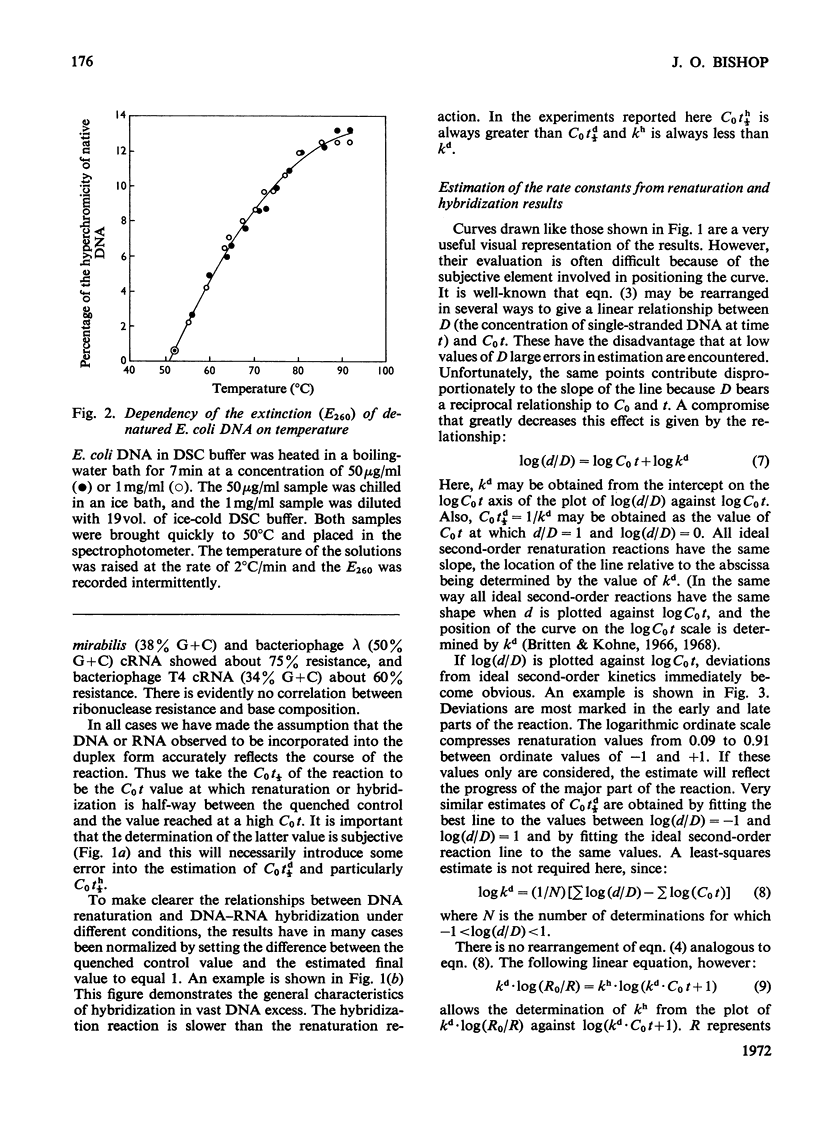
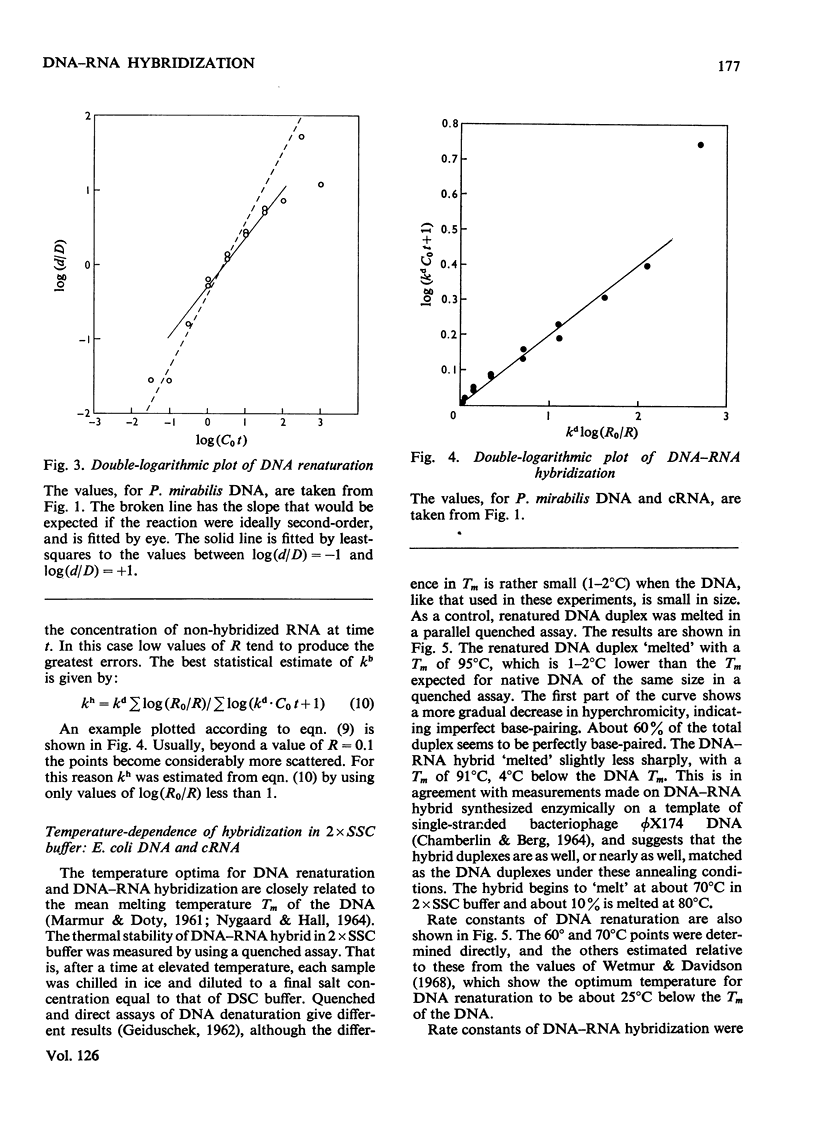
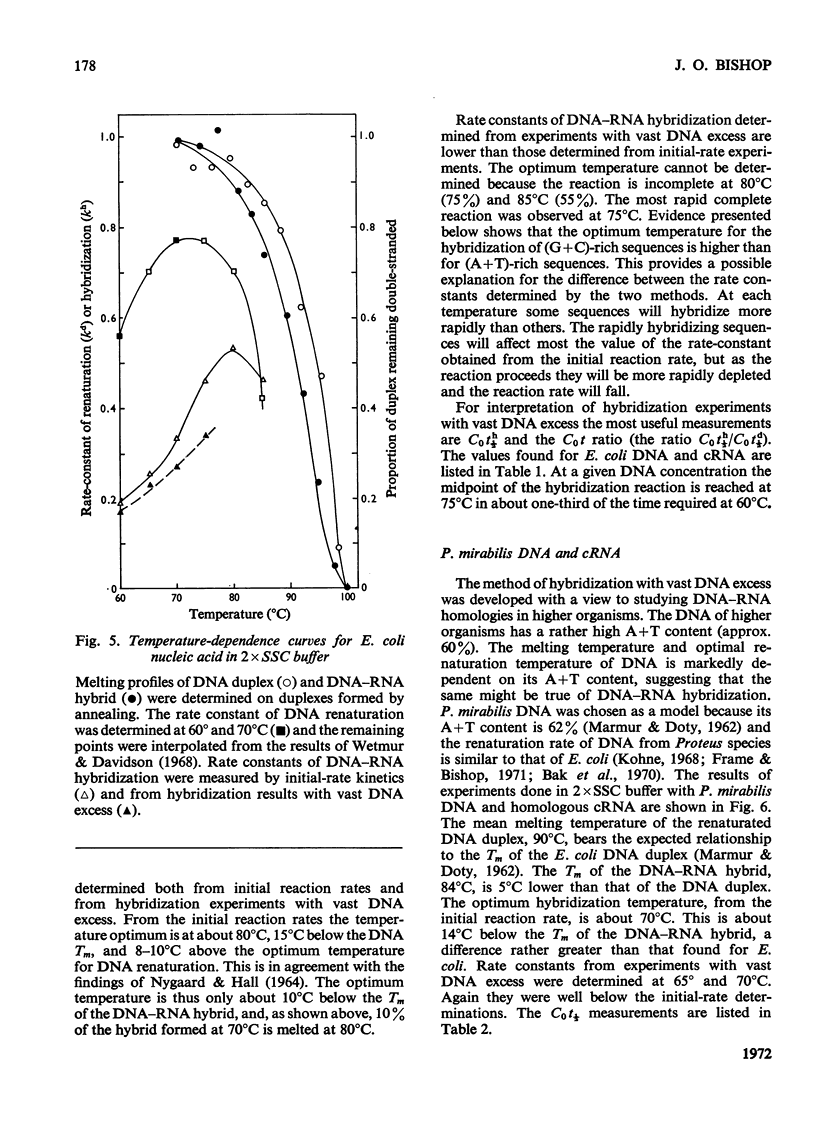
When RNA is annealed in solution with a sufficiently large excess of DNA, the kinetics of DNA–RNA hybridization are relatively simple. Methods are described for following the course of both DNA renaturation and DNA–RNA hybridization in this system. To explore the characteristics of the reaction a series of model systems was used. Each one utilized DNA (sheared to constant size) from a bacterium or bacteriophage and homologous cRNA, i.e. RNA synthesized in vitro on a template of the same DNA. Temperature optima were determined for the hybridization of Escherichia coli nucleic acids in 2×SSC and 3×SSC−50% formamide buffers, and of Proteus mirabilis nucleic acids in 2×SSC buffer. Rate-constants for DNA–RNA hybridization were measured by two methods. These gave somewhat different results, but in all cases the rate-constant of DNA–RNA hybridization was clearly less than that of DNA renaturation. Thus hybridization is a slower reaction than DNA renaturation. Nevertheless, in some cases, with a high concentration of DNA and a long annealing time, 90–95% of the added RNA became resistant to ribonuclease. Experiments are described which show that it is possible to deduce the analytical complexity of DNA with reasonable accuracy from its hybridization with complementary RNA. Similarly, it is possible to estimate the reiteration frequency of multiple DNA sequences (such as ribosomal DNA) from the hybridization of the total DNA with RNA complementary to the multiple sequences. The effect on the system of various DNA/RNA ratios from 100 to 1 is described.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Doty P. Characterization of a naturally occurring, cross-linked fraction of DNA. 1. Nature of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):379–403. doi: 10.1016/0022-2836(68)90017-x. [DOI] [PubMed] [Google Scholar]
- Attardi G., Huang P. C., Kabat S. Recognition of ribosomal RNA sites in DNA. I. Analysis of the E. coli system. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1490–1498. doi: 10.1073/pnas.53.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Parnas H., Attardi B. Pattern of RNA synthesis in duck erythrocytes in relationship to the stage of cell differentiation. Exp Cell Res. 1970 Sep;62(1):11–31. doi: 10.1016/0014-4827(79)90505-6. [DOI] [PubMed] [Google Scholar]
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. Interpretation of DNA-RNA hybridization data. Nature. 1969 Nov 8;224(5219):600–603. doi: 10.1038/224600a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. The effect of genetic complexity on the time-course of ribonucleic acid-deoxyribonucleic acid hybridization. Biochem J. 1969 Aug;113(5):805–811. doi: 10.1042/bj1130805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J., Kung G., Bekhor I. A method for the hybridization of nucleic acid molecules at low temperature. Biochemistry. 1967 Dec;6(12):3650–3653. doi: 10.1021/bi00864a005. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: FORMATION OF DNA-RNA HYBRIDS WITH SINGLE-STRANDED TEMPLATES. J Mol Biol. 1964 Feb;8:297–313. doi: 10.1016/s0022-2836(64)80139-x. [DOI] [PubMed] [Google Scholar]
- Doty P., Marmur J., Eigner J., Schildkraut C. STRAND SEPARATION AND SPECIFIC RECOMBINATION IN DEOXYRIBONUCLEIC ACIDS: PHYSICAL CHEMICAL STUDIES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):461–476. doi: 10.1073/pnas.46.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Jones I. G. Mitochondrial deoxyribonucleic acid from Tetrahymena pyriformis and its kinetic complexity. Biochem J. 1970 Mar;116(5):811–817. doi: 10.1042/bj1160811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame R., Bishop J. O. The number of sex-factors per chromosome in Escherichia coli. Biochem J. 1971 Jan;121(1):93–103. doi: 10.1042/bj1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P. On the factors controlling the reversibility of DNA denaturation. J Mol Biol. 1962 Jun;4:467–487. doi: 10.1016/s0022-2836(62)80103-x. [DOI] [PubMed] [Google Scholar]
- Gelderman A. H., Rake A. V., Britten R. J. Transcription of nonrepeated DNA in neonatal and fetal mice. Proc Natl Acad Sci U S A. 1971 Jan;68(1):172–176. doi: 10.1073/pnas.68.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- Kohne D. E. Isolation and characterization of bacterial ribosomal RNA cistrons. Biophys J. 1968 Oct;8(10):1104–1118. doi: 10.1016/S0006-3495(68)86542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird C. D., McCarthy B. J. Magnitude of interspecific nucleotide sequence variability in Drosophila. Genetics. 1968 Oct;60(2):303–322. doi: 10.1093/genetics/60.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Thermal renaturation of deoxyribonucleic acids. J Mol Biol. 1961 Oct;3:585–594. doi: 10.1016/s0022-2836(61)80023-5. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Melli M., Bishop J. O. Hybridization between rat liver DNA and complementary RNA. J Mol Biol. 1969 Feb 28;40(1):117–136. doi: 10.1016/0022-2836(69)90300-3. [DOI] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- ROBINSON W. S., HSU W. I., FOX C. F., WEISS S. B. ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID. IV. THE DEOXYRIBONUCLEIC ACID-DIRECTED SYNTHESIS OF COMPLEMENTARY CYTOPLASMIC RIBONUCLEIC ACID COMPONENTS. J Biol Chem. 1964 Sep;239:2944–2951. [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L. Messenger RNA in avian erythroblasts at the transcriptional and translational levels and the problem of regulation in animal cells. J Cell Physiol. 1968 Oct;72(2 Suppl):181+–181+. doi: 10.1002/jcp.1040720413. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Conformational changes of single-stranded DNA. J Mol Biol. 1969 Apr;41(2):189–197. doi: 10.1016/0022-2836(69)90384-2. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


