Abstract
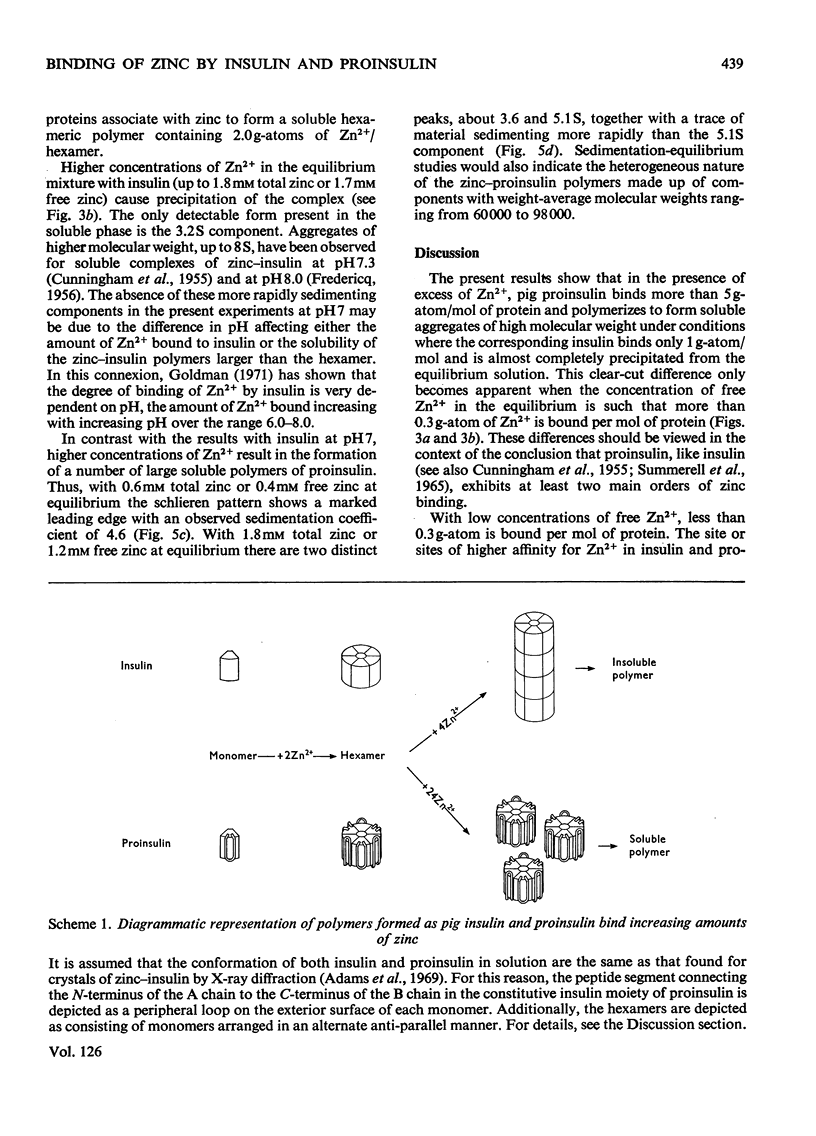
1. The reversible interaction of zinc with pig insulin and proinsulin has been studied at pH7 by equilibrium dialysis (ultrafiltration) and by sedimentation equilibrium and velocity measurements in the ultracentrifuge. Binding values calculated from equilibria, where the ratio of free to bound zinc was varied in the range 0.01:1–10:1, indicated that proinsulin and insulin each contained two main orders of zinc binding with very different affinities for the metal. 2. In equilibria containing low concentrations of free zinc (free: bound ratios of 0.01–0.1:1) both insulin and proinsulin aggregated to form soluble hexamers containing firmly bound zinc (up to 0.284g-atom/monomer) with an apparent intrinsic association constant of 1.9×106m−1. 3. Higher concentrations of zinc (free: bound ratios of 0.1–10.0:1) resulted in a progressive difference in the zinc binding, aggregation and solubility properties of the metal complexes of insulin and proinsulin. At the highest concentration of free zinc, proinsulin bound a total of more than 5.0g-atom/monomer and aggregated to form a mixture of soluble polymers (mainly 5.1S). In contrast, insulin bound a total of only 1.0g-atom/monomer and was almost completely precipitated from solution. 4. These results would indicate that the presence of the peptide segment connecting the insulin moiety in proinsulin does not prevent the firm binding of zinc to the insulin moiety and the formation of hexamers of zinc–proinsulin. At the same time although the connecting peptide contains additional sites of lower affinity for zinc, which should facilitate inter- and intra-molecular cross-linking, the general conformation of the zinc–proinsulin hexamer must preclude the formation of very large and close-packed aggregates that are insoluble in solutions at equilibrium.
Full text
PDF

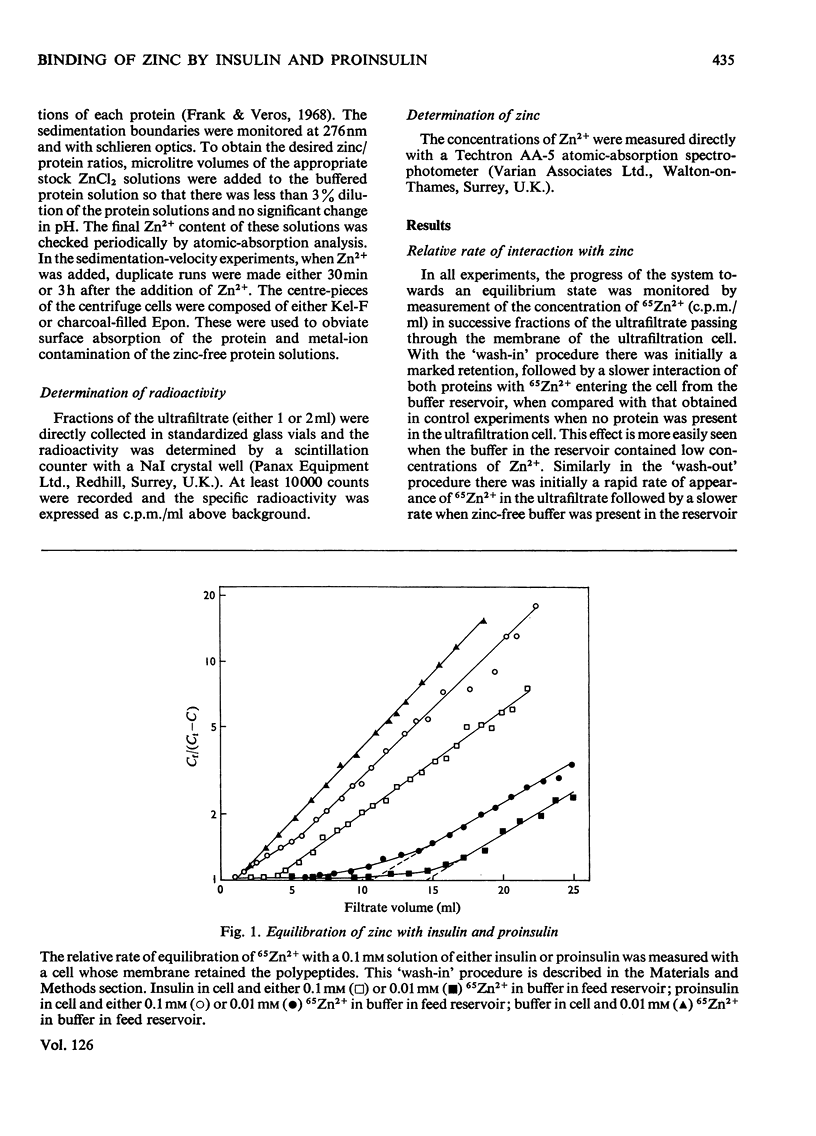
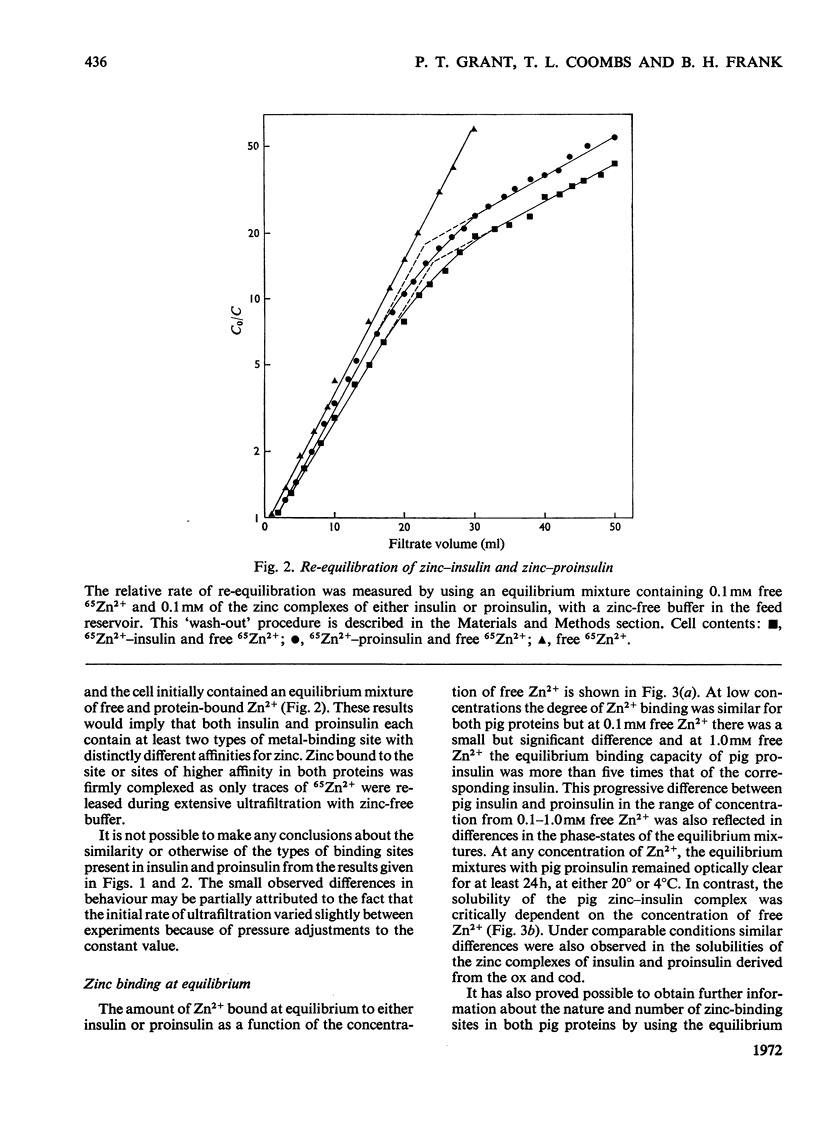
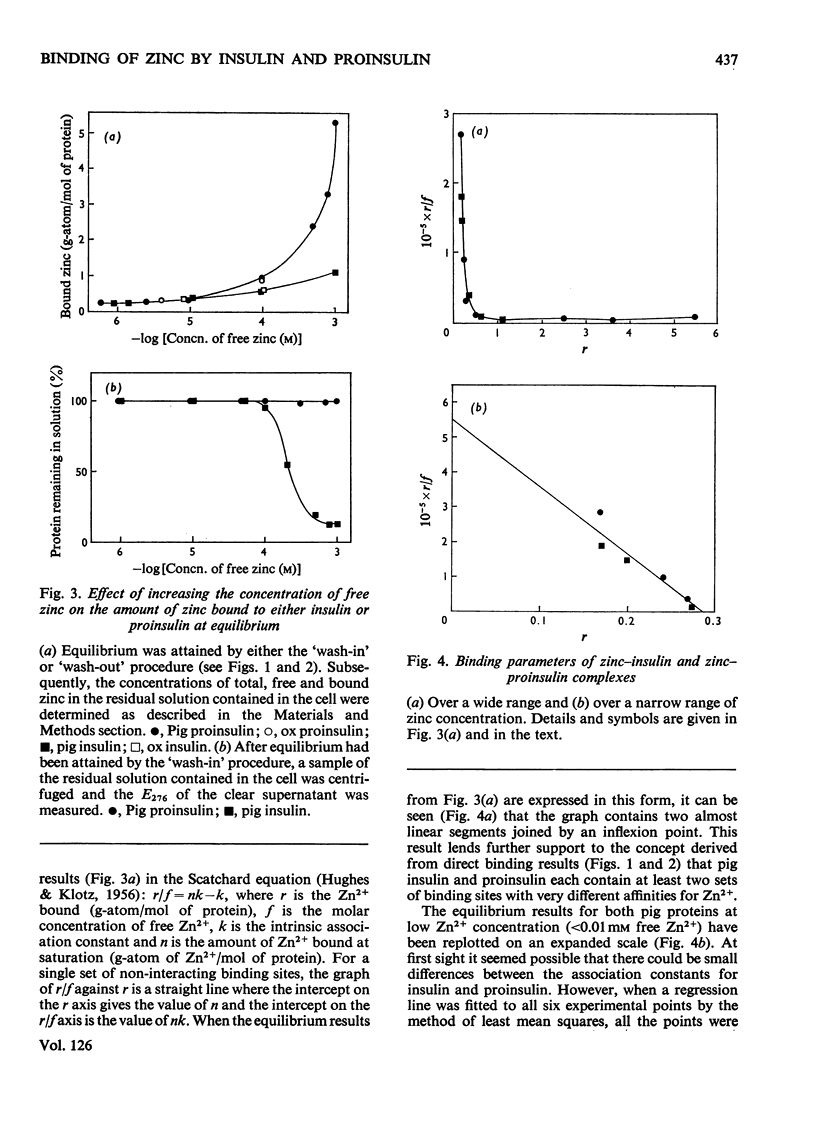
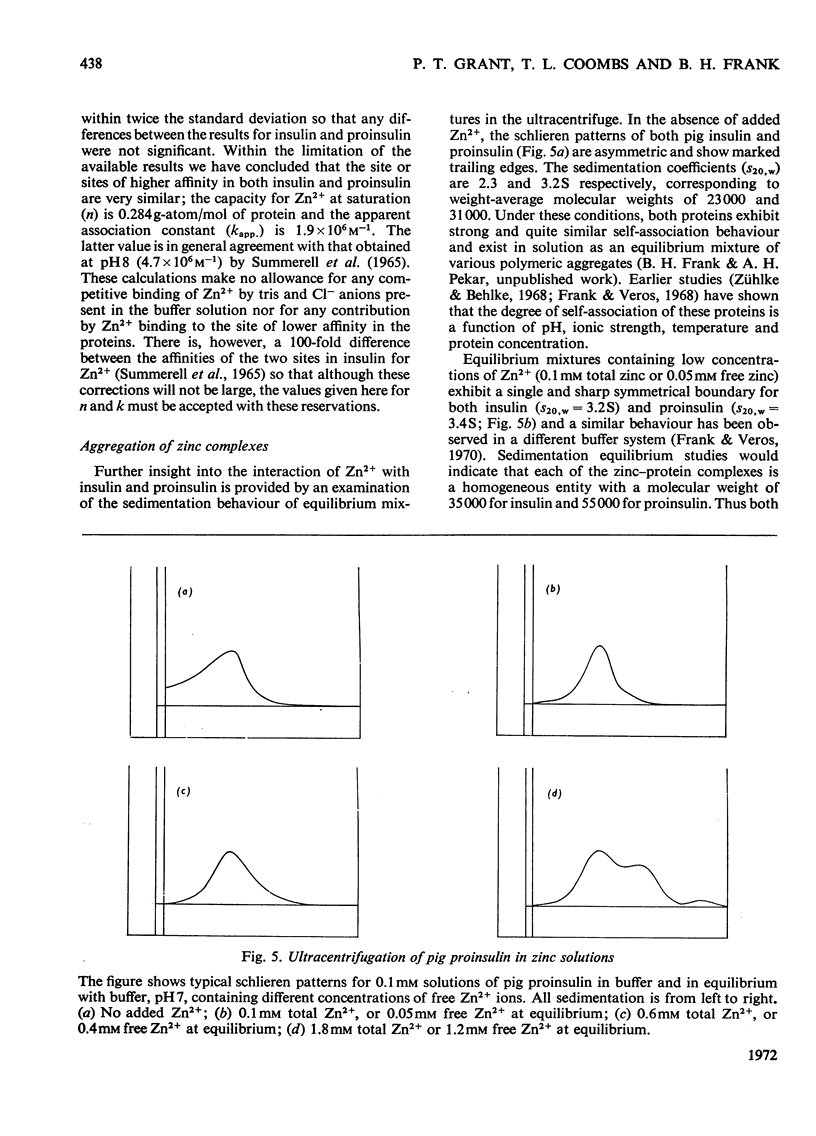

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt W. F., Robinson S. M., Bixler H. J. Membrane ultrafiltration: the diafiltration technique and its application to microsolute exchange and binding phenomena. Anal Biochem. 1968 Oct 10;26(1):151–173. doi: 10.1016/0003-2697(68)90039-0. [DOI] [PubMed] [Google Scholar]
- Bromer W. W., Chance R. E. Preparation and characterization of desoctapeptide-insulin. Biochim Biophys Acta. 1967 Feb 21;133(2):219–223. doi: 10.1016/0005-2795(67)90061-x. [DOI] [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- FREDERICQ E. The association of insulin molecular units in aqueous solutions. Arch Biochem Biophys. 1956 Nov;65(1):218–228. doi: 10.1016/0003-9861(56)90189-8. [DOI] [PubMed] [Google Scholar]
- Frank B. H., Veros A. J. Interaction of zinc with proinsulin. Biochem Biophys Res Commun. 1970 Jan 23;38(2):284–289. doi: 10.1016/0006-291x(70)90710-2. [DOI] [PubMed] [Google Scholar]
- Frank B. H., Veros A. J. Physical studies on proinsulin-association behavior and conformation in solution. Biochem Biophys Res Commun. 1968 Jul 26;32(2):155–160. doi: 10.1016/0006-291x(68)90362-8. [DOI] [PubMed] [Google Scholar]
- Fullerton W. W., Potter R., Low B. W. Proinsulin: Crystallization and preliminary x-ray diffraction studies. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1213–1219. doi: 10.1073/pnas.66.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. T., Coombs T. L. Proinsulin, a biosynthetic precursor of insulin. Essays Biochem. 1970;6:69–92. [PubMed] [Google Scholar]
- Grant P. T., Reid K. B. Biosynthesis of an insulin precursor by islet tissue of cod (Gadus callarias). Biochem J. 1968 Nov;110(2):281–288. doi: 10.1042/bj1100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider M. H., Howell S. L., Lacy P. E. Isolation and properties of secretory granules from rat islets of Langerhans. II. Ultrastructure of the beta granule. J Cell Biol. 1969 Apr;41(1):162–166. doi: 10.1083/jcb.41.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES T. R., KLOTZ I. M. Analysis of metal-protein complexes. Methods Biochem Anal. 1956;3:265–299. doi: 10.1002/9780470110195.ch9. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F. Conversion of proinsulin to insulin in a subcellular fraction from rat islets. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1223–1230. doi: 10.1016/0006-291x(70)90217-2. [DOI] [PubMed] [Google Scholar]
- Yip C. C. A bovine pancreatic enzyme catalyzing the conversion of proinsulin to insulin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1312–1315. doi: 10.1073/pnas.68.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zühlke H., Behlke J. Fraktionierung handelsüblichen insulins. Das verhalten von proinsulin in der ultrazentrifuge. FEBS Lett. 1968 Dec;2(2):130–132. doi: 10.1016/0014-5793(68)80122-x. [DOI] [PubMed] [Google Scholar]


