Abstract
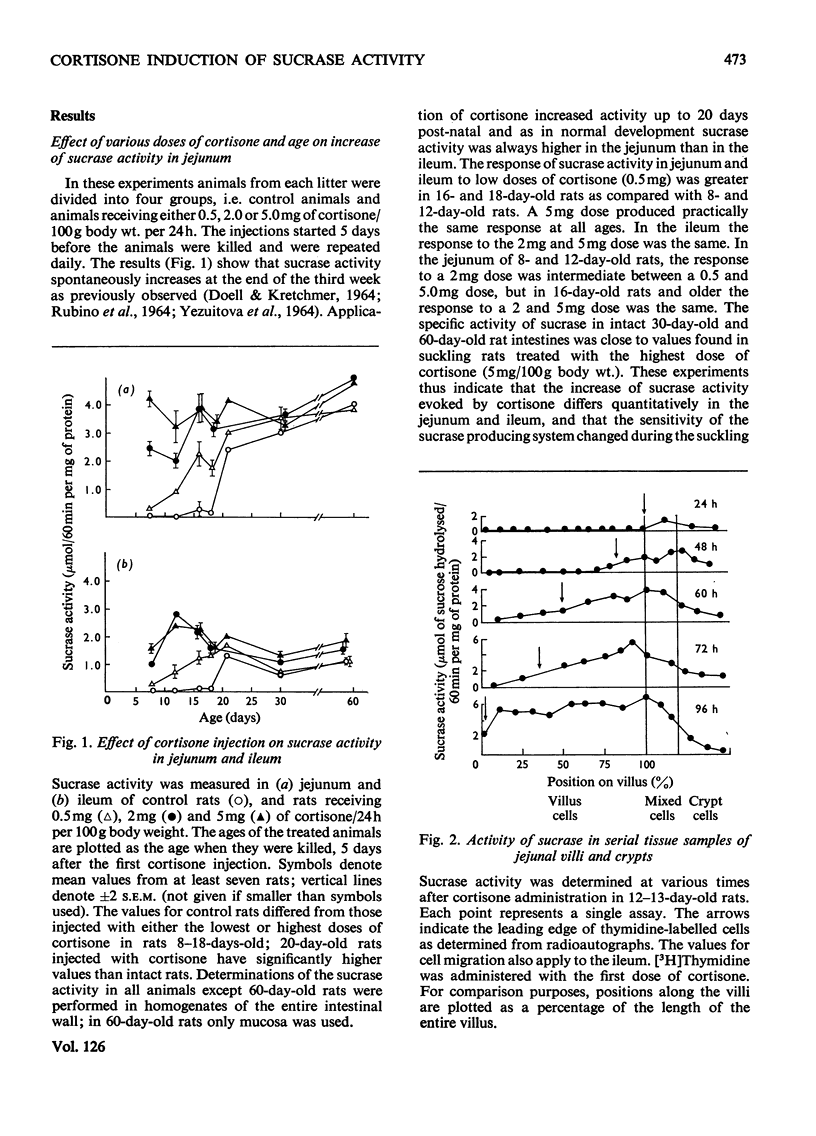
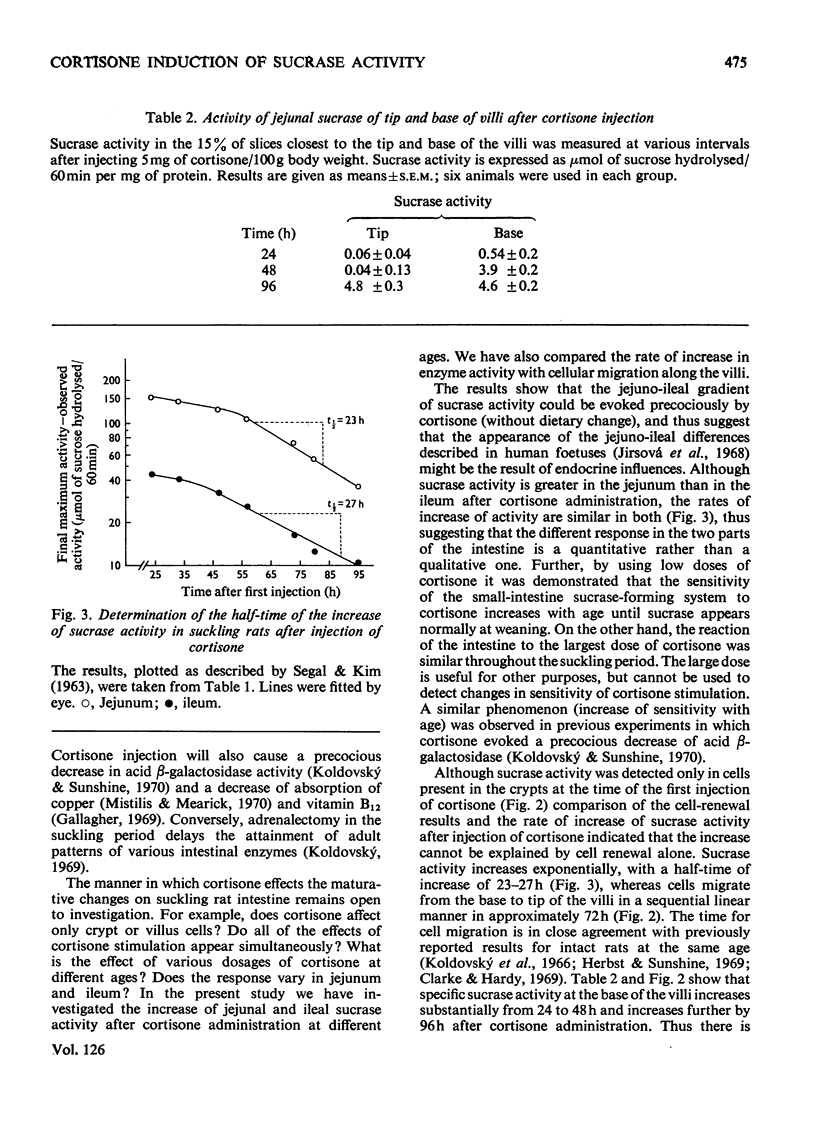
The increase of sucrase activity in homogenates of jejunum and ileum of suckling rats after cortisone administration has been investigated. Serial tissue sections of villi and crypts were also assayed for sucrase activity and these results were compared with the migration of cells labelled with [3H]thymidine along the villus. By using a low dose of cortisone (0.5mg/day per 100g body wt.) it was found that the sensitivity of the small intestine producing system to cortisone stimulation increased during the suckling period. On the other hand, 5mg of cortisone/day per 100g body wt. produced practically the same increase of sucrase during the entire suckling period. Sucrase activity in homogenates of the entire small-intestinal wall was first detected 24h after the first injection of cortisone (5mg/day per 100g body weight) to 9-day-old animals and maximum activity both in the jejunum and ileum was reached by 120h. Jejunal activity was greater than ileal activity, but the rate of the increase was similar. The half-time of the increase was 23–27h, whereas enterocytes migrate from the base to the tip of the villi in approximately 72h. Comparison of sucrase activity in serial tissue sections of villi and crypts at various times after cortisone treatment showed that the leading edge of sucrase activity proceeds toward the tip of the villi at the same rate as the advancing edge of newly formed cells. Sucrase activity increased in the newly induced cells as they migrated to the tip of the villi. It was concluded that the increase of sucrase activity in suckling rats after cortisone stimulation is due to at least three factors: (1) increase of activity in newly differentiating cells, (2) increased percentage of villus cells with sucrase activity and (3) continued production or activation of sucrase activity as the cells migrate along the villi.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asp N. G., Koldovský O., Hosková J. Use of the glucoseoxidase method for assay of disaccharidase activities in the small intestin--a limitation. Physiol Bohemoslov. 1967;16(6):508–511. [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Some comments on the histochemical characterization of muscle adenosine triphosphatase. J Histochem Cytochem. 1969 Jun;17(6):431–432. doi: 10.1177/17.6.431. [DOI] [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M., Hardy R. N. An analysis of the mechanism of cessation of uptake of macromolecular substances by the intestine of the young rat ('closure'). J Physiol. 1969 Sep;204(1):127–134. doi: 10.1113/jphysiol.1969.sp008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Doell R. G., Rosen G., Kretchmer N. Immunochemical studies of intestinal disaccharidases during normal and precocious development. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1268–1273. doi: 10.1073/pnas.54.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher N. D. Mechanism and site of vitamin B 12 absorption in suckling rats. Nature. 1969 May 31;222(5196):877–878. doi: 10.1038/222877a0. [DOI] [PubMed] [Google Scholar]
- HALLIDAY R. The effect of steroid hormones on the absorption of antibody by the young rat. J Endocrinol. 1959 Jan;18(1):56–66. doi: 10.1677/joe.0.0180056. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Sunshine P. Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr Res. 1969 Jan;3(1):27–33. doi: 10.1203/00006450-196901000-00004. [DOI] [PubMed] [Google Scholar]
- James W. P., Alpers D. H., Gerber J. E., Isselbacher K. J. The turnover of disaccharidases and brush border proteins in rat intestine. Biochim Biophys Acta. 1971 Feb 23;230(2):194–203. doi: 10.1016/0304-4165(71)90204-2. [DOI] [PubMed] [Google Scholar]
- Jirsová V., Koldovský O., Heringová A., Uher J., Jodl J. Development of invertase activity in the intestines of human fetuses, appearance of jejunoileal differences. Biol Neonat. 1968;13(3):143–146. doi: 10.1159/000240141. [DOI] [PubMed] [Google Scholar]
- Koldovsky O., Sunshine P., Kretchmer N. Cellular migration of intestinal epithelia in suckling and weaned rats. Nature. 1966 Dec 17;212(5068):1389–1390. doi: 10.1038/2121389a0. [DOI] [PubMed] [Google Scholar]
- Koldovský O., Herbst J. J., Burke J., Sunshine P. RNA and DNA in intestinal mucosa during development of normal and cortisone-treated rats. Growth. 1970 Dec;34(4):359–367. [PubMed] [Google Scholar]
- Koldovský O., Sunshine P. Effect of cortisone on the developmental pattern of the neutral and the acid beta-galactosidase of the small intestine of the rat. Biochem J. 1970 Apr;117(3):467–471. doi: 10.1042/bj1170467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPKIN M., QUASTLER H. Studies of protein metabolism in intestinal epithelial cells. J Clin Invest. 1962 Mar;41:646–653. doi: 10.1172/JCI104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- Overton J. Fine structure of the free cell surface in developing mouse intestinal mucosa. J Exp Zool. 1965 Jul;159(2):195–201. doi: 10.1002/jez.1401590205. [DOI] [PubMed] [Google Scholar]
- RUBINO A., ZIMBALATTI F., AURICCHIO S. INTESTINAL DISACCHARIDASE ACTIVITIES IN ADULT AND SUCKLING RATS. Biochim Biophys Acta. 1964 Nov 22;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- SEGAL H. L., KIM Y. S. GLUCOCORTICOID STIMULATION OF THE BIOSYNTHESIS OF GLUTAMIC-ALANINE TRANSAMINASE. Proc Natl Acad Sci U S A. 1963 Nov;50:912–918. doi: 10.1073/pnas.50.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]


