Abstract
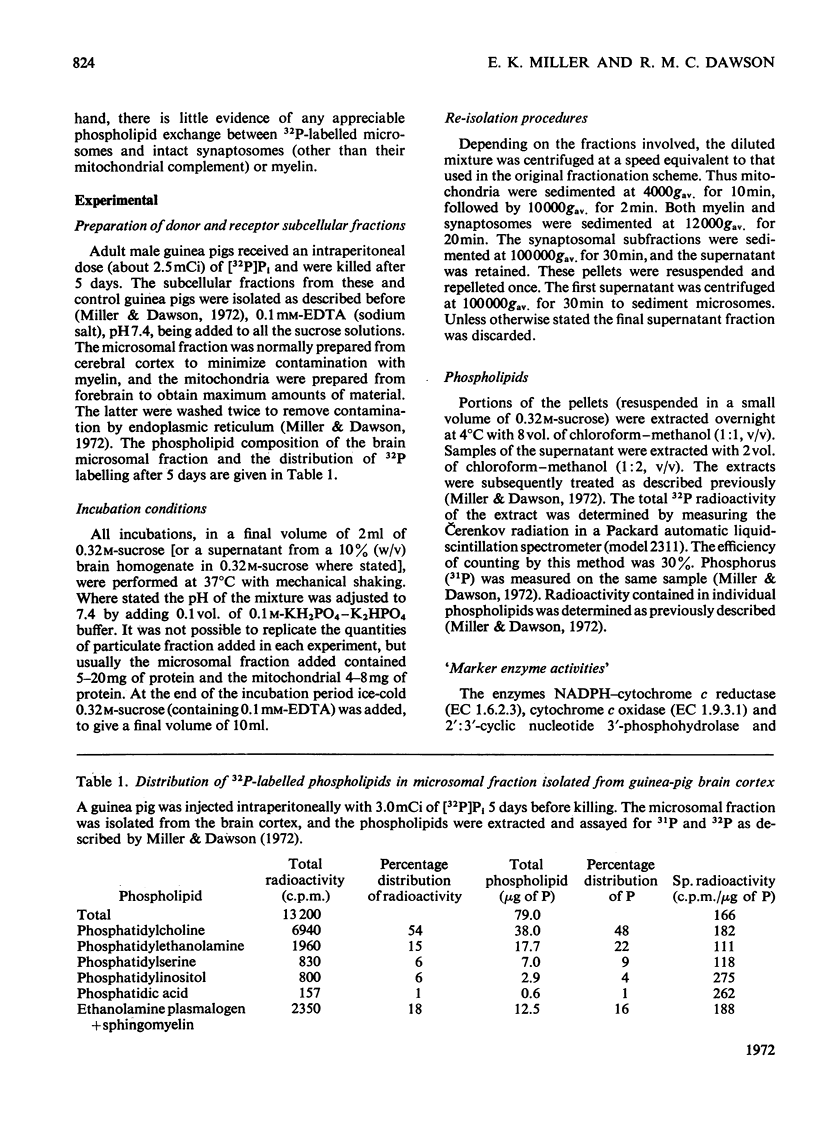
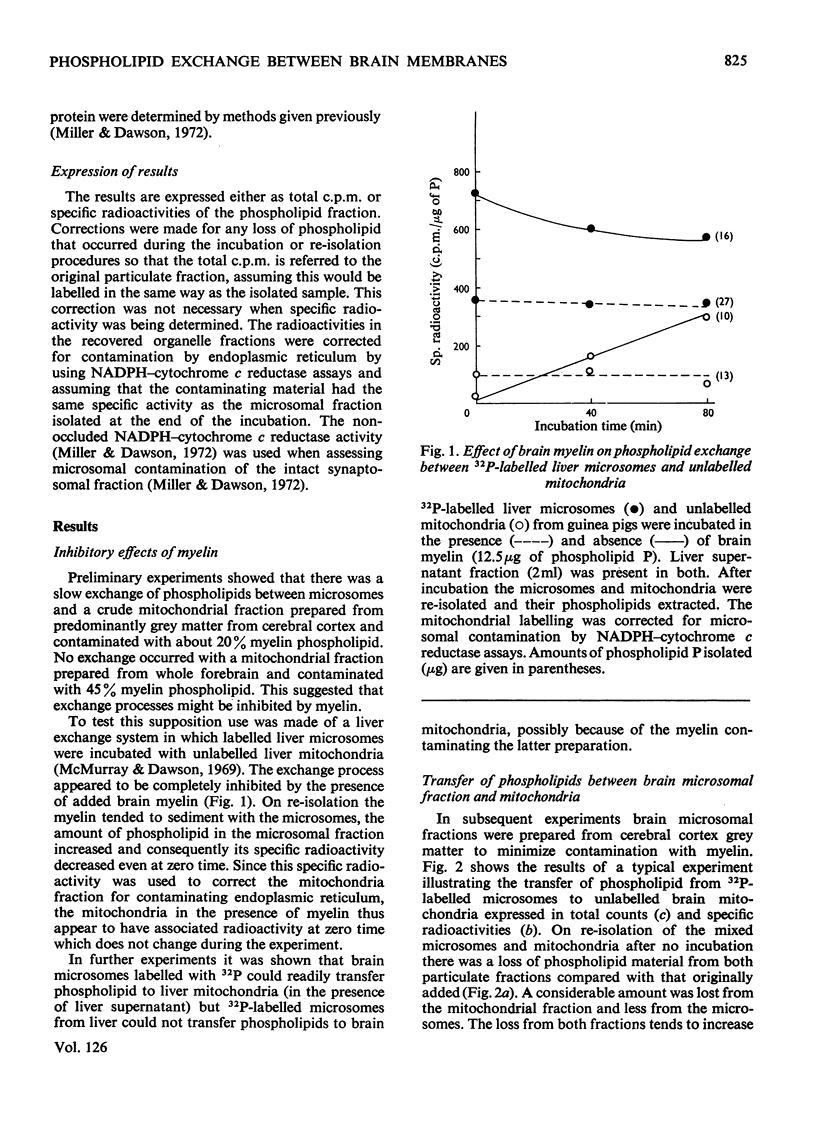
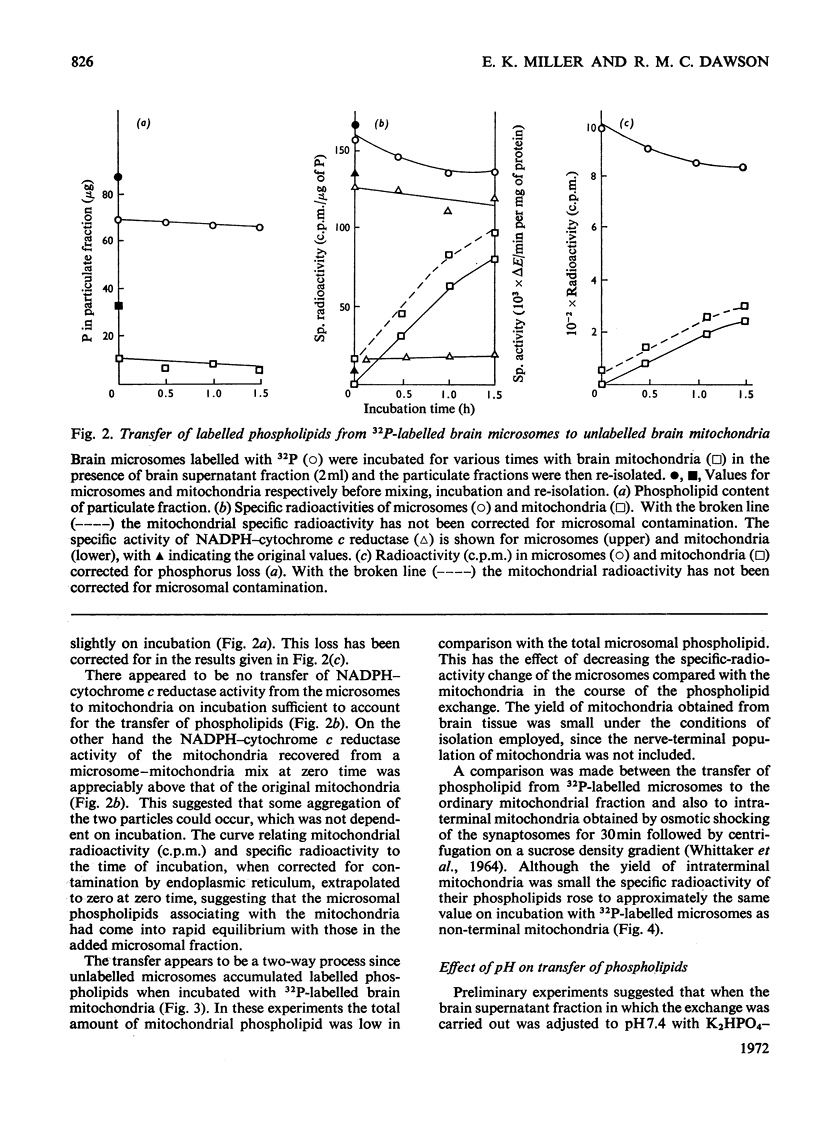
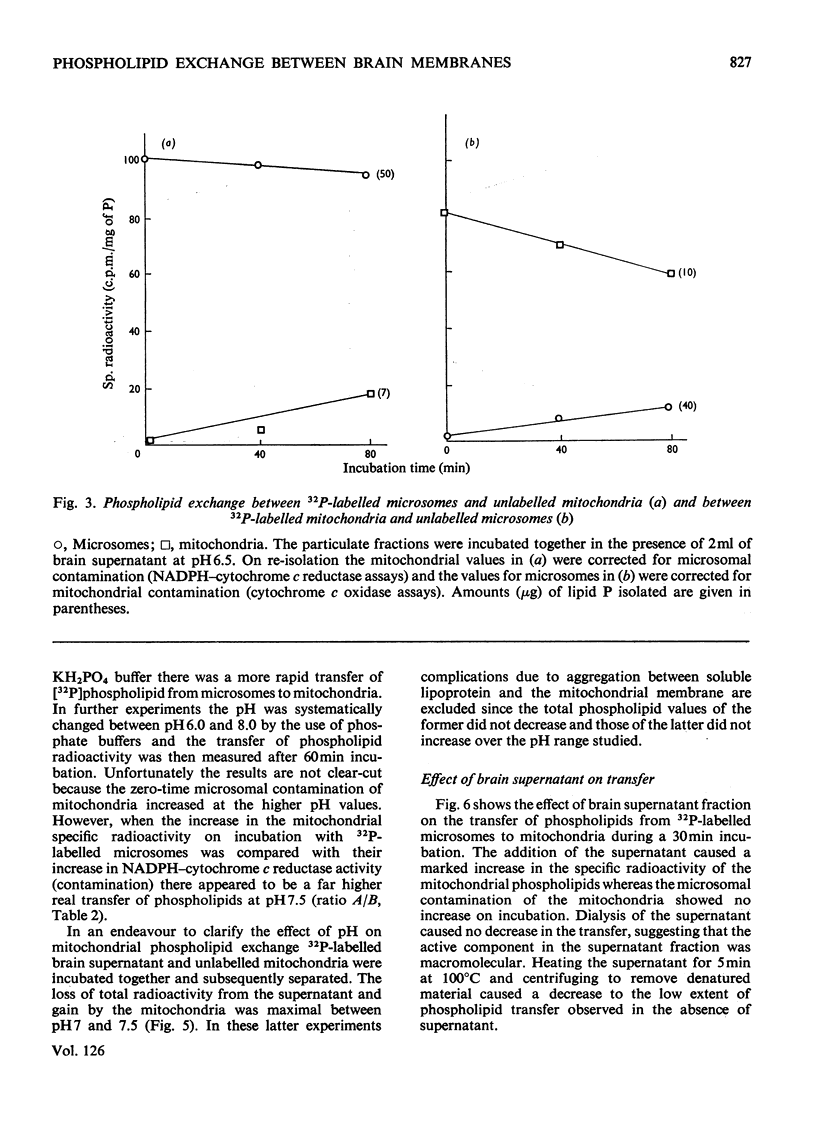
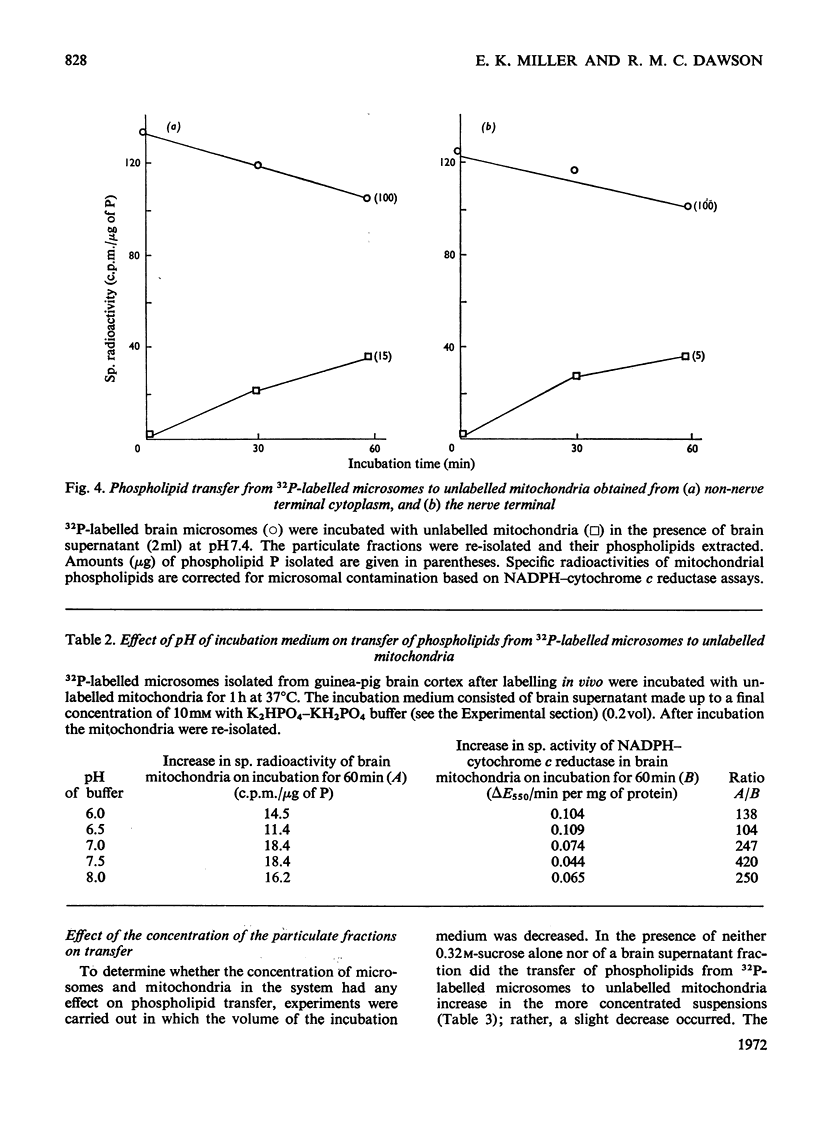
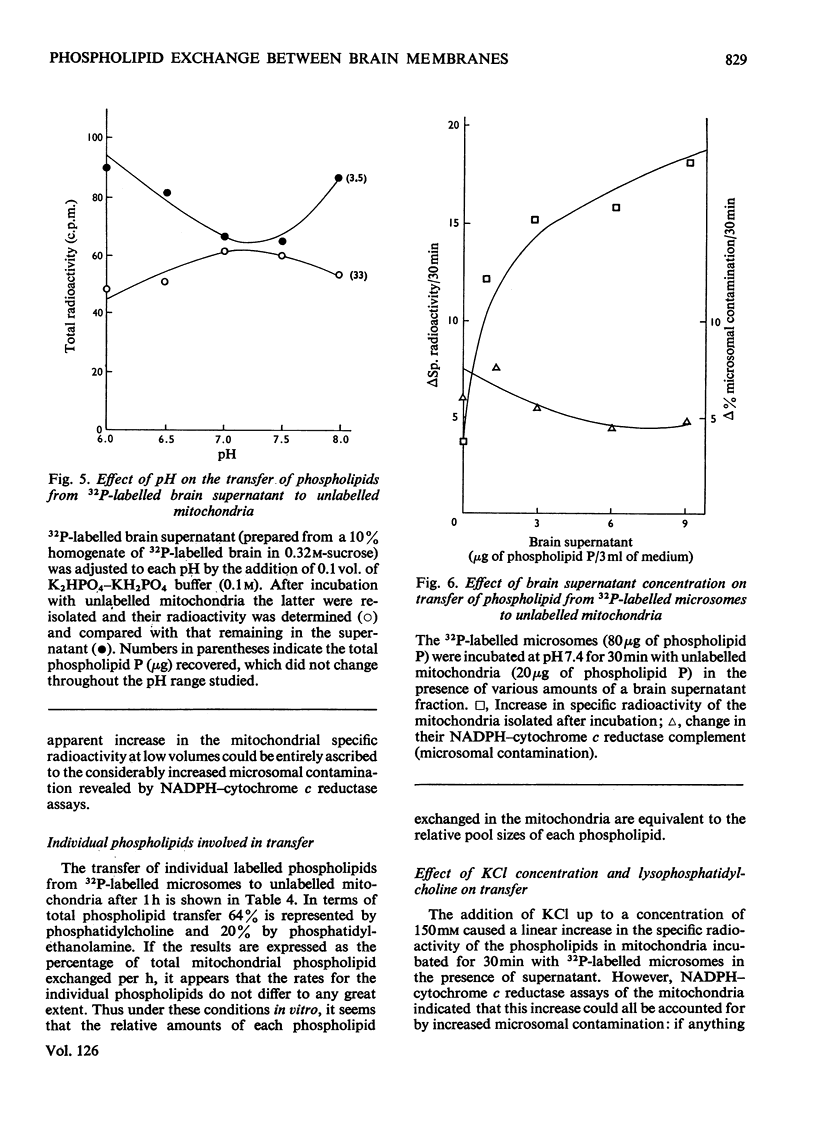
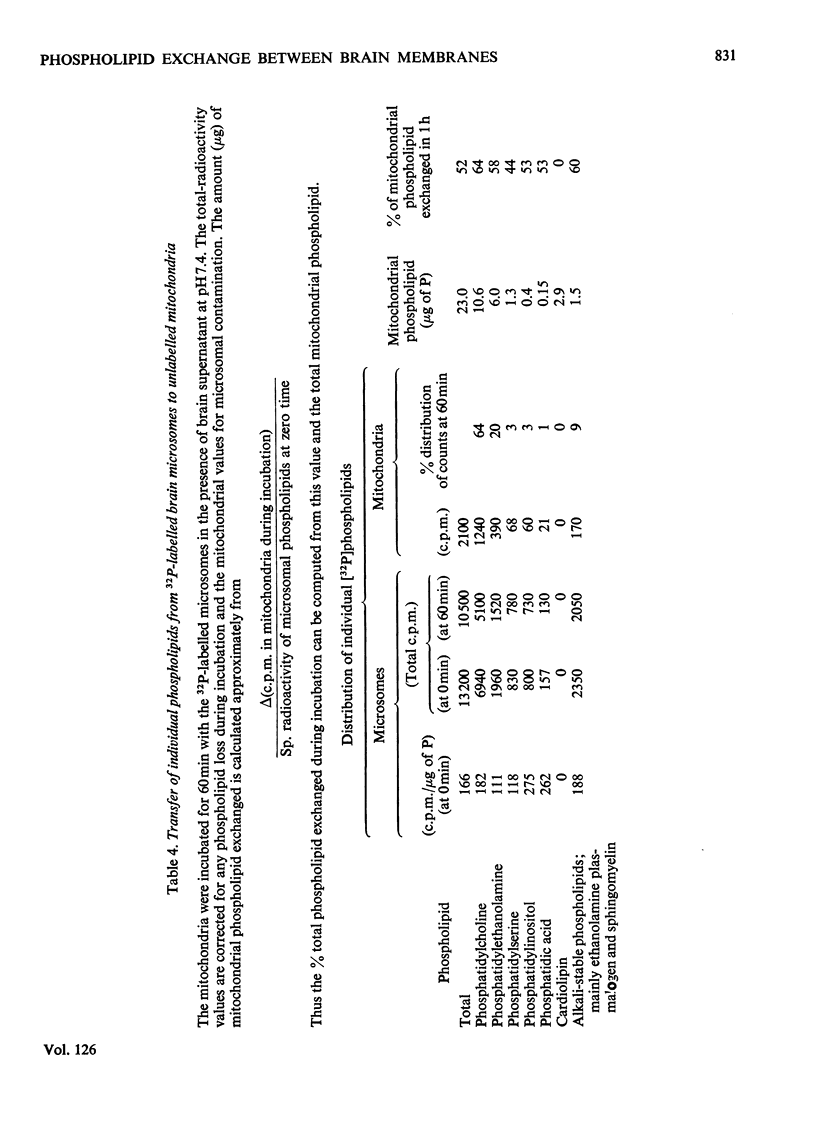
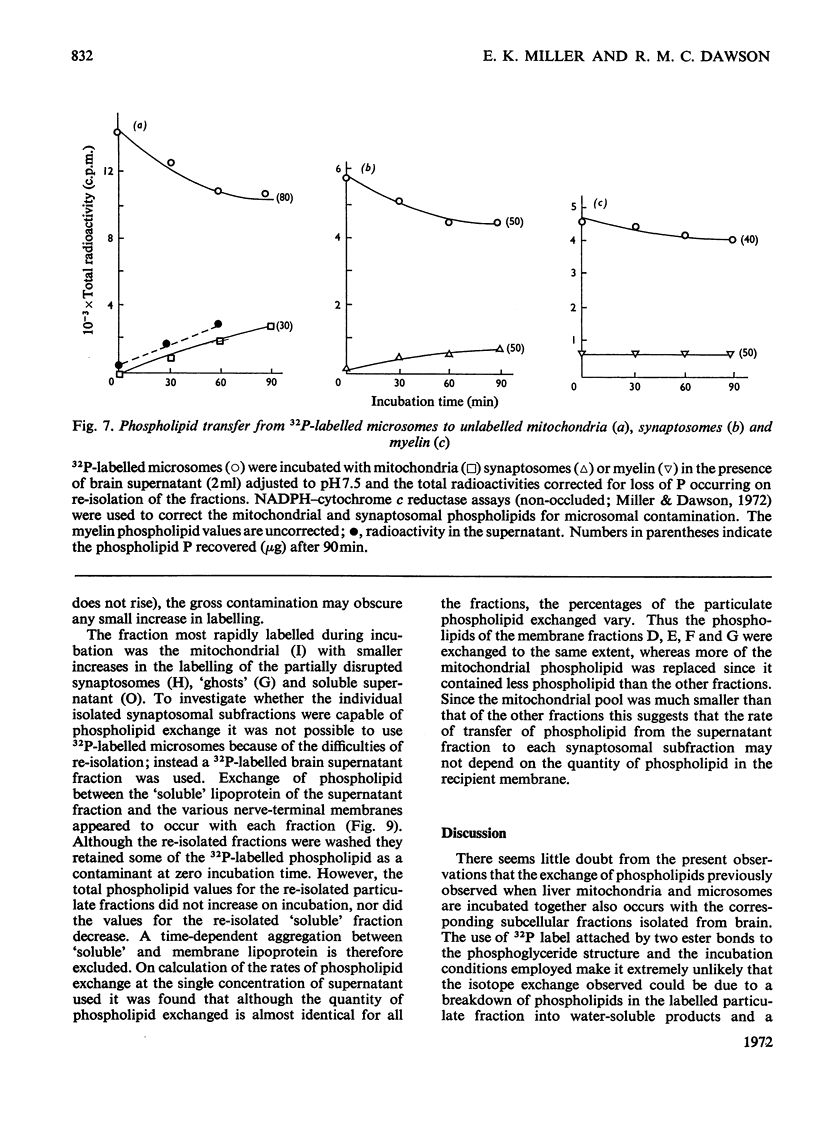
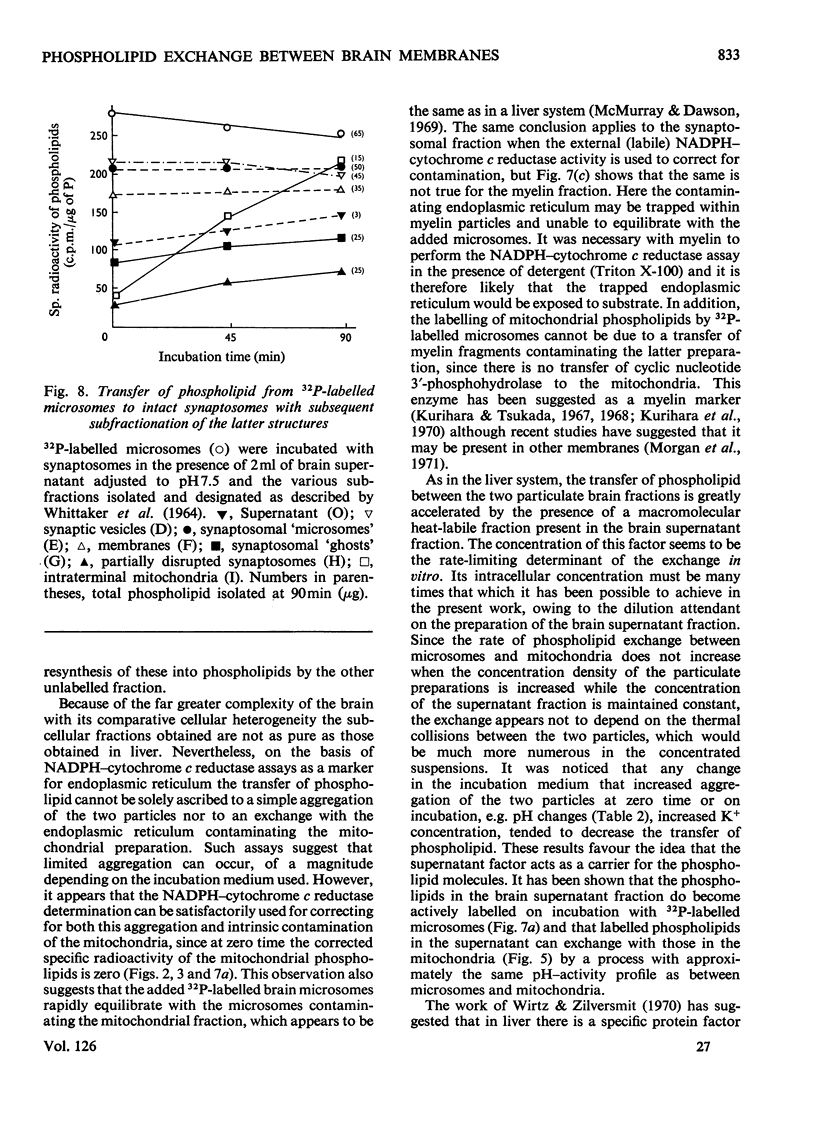
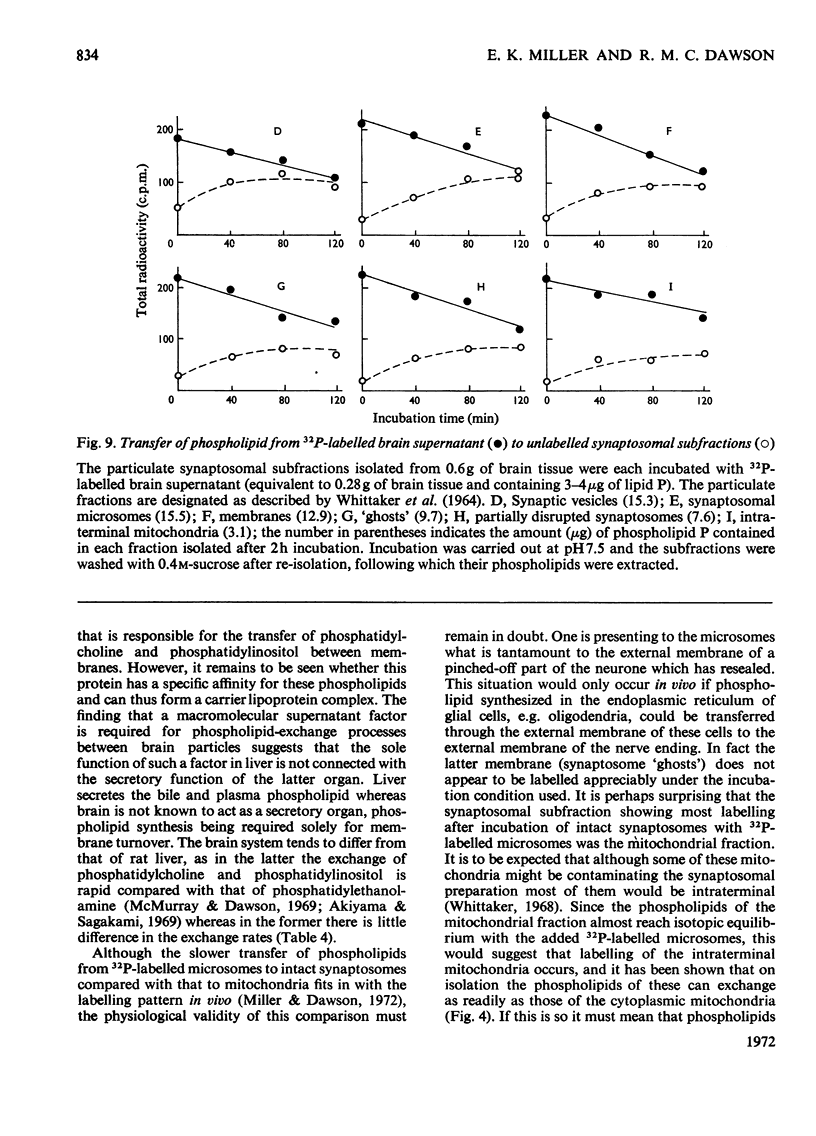
1. When unlabelled mitochondria from guinea-pig brain were incubated with a 32P-labelled microsomal fraction from brain there was a transfer of phospholipid to the mitochondria, which could not be accounted for by an aggregation of microsomes and mitochondria or an exchange with microsomes contaminating the mitochondria. Under similar circumstances there was a transfer of phospholipid from 32P-labelled mitochondria to microsomes, indicating that the process was one of exchange. 2. The transfer from microsomes was greatly stimulated by a non-dialysable heat-labile macromolecular component in the brain supernatant fraction but not by the concentration of the particulate fractions. 3. Phospholipid-exchange processes occurred most readily between pH7 and 7.5 and were inhibited by the presence of myelin and on the addition of lysophosphatidylcholine. 4. The rates of transfer of individual phospholipids from brain microsomes to mitochondria were similar. 5. 32P-labelled microsomes could slowly donate phospholipid to the isolated synaptosomal (nerve-ending) fraction but the phospholipids of the myelin fraction did not exchange. 6. Subfractionation of the synaptosomal fraction after [32P]phospholipid transfer showed that the mitochondria were most actively labelled during the incubation. All of the isolated individual synaptosomal membranes were capable of acquiring phospholipid on incubation with a 32P-labelled brain supernatant fraction although a greater percentage was again exchanged by the mitochondrial fraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama M., Sakagami T. Exchange of mitochondrial lecithin and cephalin with those in rat liver microsomes. Biochim Biophys Acta. 1969 Jul 29;187(1):105–112. [PubMed] [Google Scholar]
- Jungalwala F. B., Dawson R. M. Phospholipid synthesis and exchange in isolated liver cells. Biochem J. 1970 Apr;117(3):481–490. doi: 10.1042/bj1170481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungalwala F. B., Dawson R. M. The turnover of myelin phospholipids in the adult and developing rat brain. Biochem J. 1971 Aug;123(5):683–693. doi: 10.1042/bj1230683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Nussbaum J. L., Mandel P. 2',3'-cyclic nucleotide 3'-phosphohydrolase in brains of mutant mice with deficient myelination. J Neurochem. 1970 Jul;17(7):993–997. doi: 10.1111/j.1471-4159.1970.tb02252.x. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Tsukada Y. The regional and subcellular distribution of 2',3'-cyclic nucleotide 3'-phosphohydrolase in the central nervous system. J Neurochem. 1967 Dec;14(12):1167–1174. doi: 10.1111/j.1471-4159.1967.tb06164.x. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Rodríguez De Lores A., De Robertis E. 32P incorporation into different membranous structures separated from rat cerebral cortex. J Neurochem. 1969 Jan;16(1):101–106. doi: 10.1111/j.1471-4159.1969.tb10347.x. [DOI] [PubMed] [Google Scholar]
- MIANI N. PROXIMO-DISTAL MOVEMENT OF PHOSPHOLIPID IN THE AXOPLASM OF THE INTACT AND REGENERATING NEURONS. Prog Brain Res. 1964;13:115–126. doi: 10.1016/s0079-6123(08)60141-7. [DOI] [PubMed] [Google Scholar]
- Mandel P., Nussbaum J. L. Incorporation of 32P into the phosphatides of myelin sheaths and of intracellular membranes. J Neurochem. 1966 Aug;13(8):629–642. doi: 10.1111/j.1471-4159.1966.tb09871.x. [DOI] [PubMed] [Google Scholar]
- McMurray W. C., Dawson R. M. Phospholipid exchange reactions within the liver cell. Biochem J. 1969 Mar;112(1):91–108. doi: 10.1042/bj1120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K., Dawson R. M. Can mitochondria and synaptosomes of guinea-pig brain synthesize phospholipids? Biochem J. 1972 Feb;126(4):805–821. doi: 10.1042/bj1260805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Law J. H. Phosphatidylcholine biosynthesis in Tetrahymena pyriformis. Biochim Biophys Acta. 1970 Feb 10;202(1):141–152. doi: 10.1016/0005-2760(70)90225-0. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Lecithin synthesis, intracellular transport, and secretion in rat liver. IV. A radioautographic and biochemical study of choline-deficient rats injected with choline-3H. J Cell Biol. 1969 Feb;40(2):461–483. doi: 10.1083/jcb.40.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P. The morphology of fractions of rat forebrain synaptosomes separated on continuous sucrose density gradients. Biochem J. 1968 Jan;106(2):412–417. doi: 10.1042/bj1060412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz K. W.A., Zilversmit D. B. Partial purification of phospholipid exchange protein from beef heart. FEBS Lett. 1970 Mar 16;7(1):44–46. doi: 10.1016/0014-5793(70)80614-7. [DOI] [PubMed] [Google Scholar]
- Wirtz K. W., Zilversmit D. B. Exchange of phospholipids between liver mitochondria and microsomes in vitro. J Biol Chem. 1968 Jul 10;243(13):3596–3602. [PubMed] [Google Scholar]
- Wirtz K. W., Zilversmit D. B. The use of phenobarbital and carbon tetrachloride to examine liver phospholipid exchange in intact rats. Biochim Biophys Acta. 1969 Dec 17;187(4):468–476. doi: 10.1016/0005-2760(69)90043-5. [DOI] [PubMed] [Google Scholar]


