Abstract
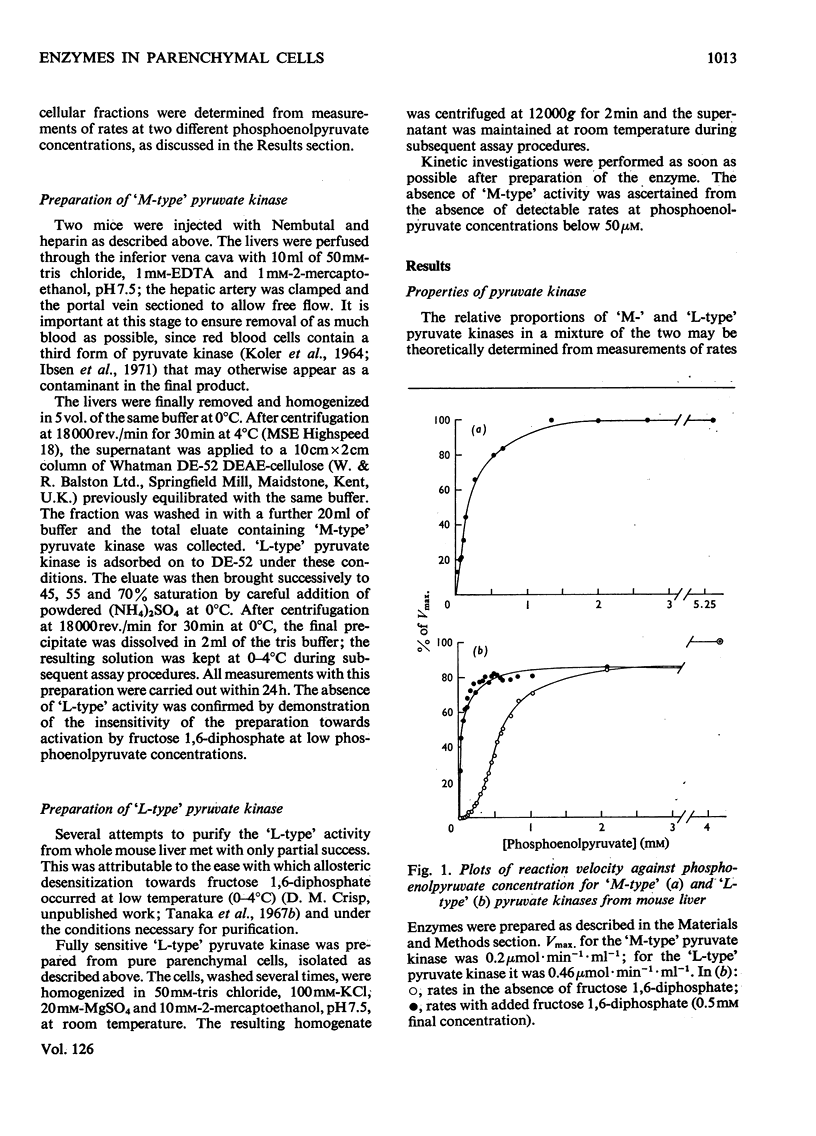
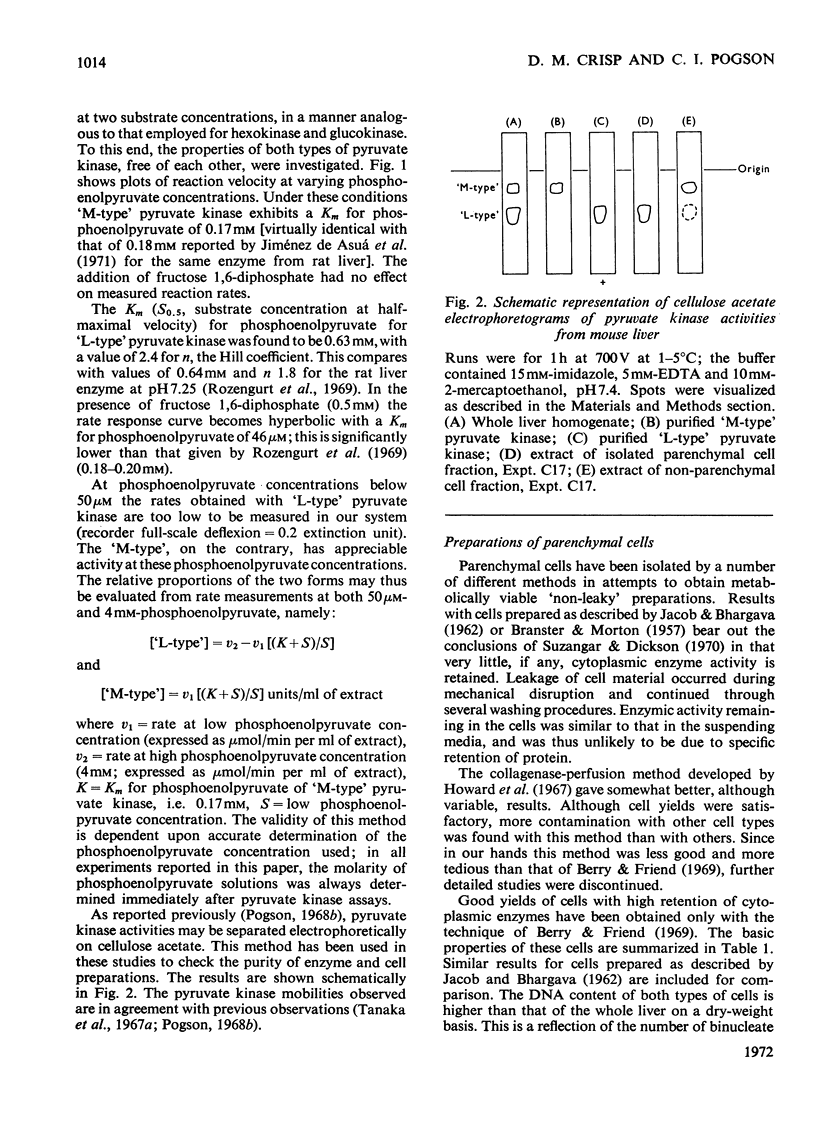
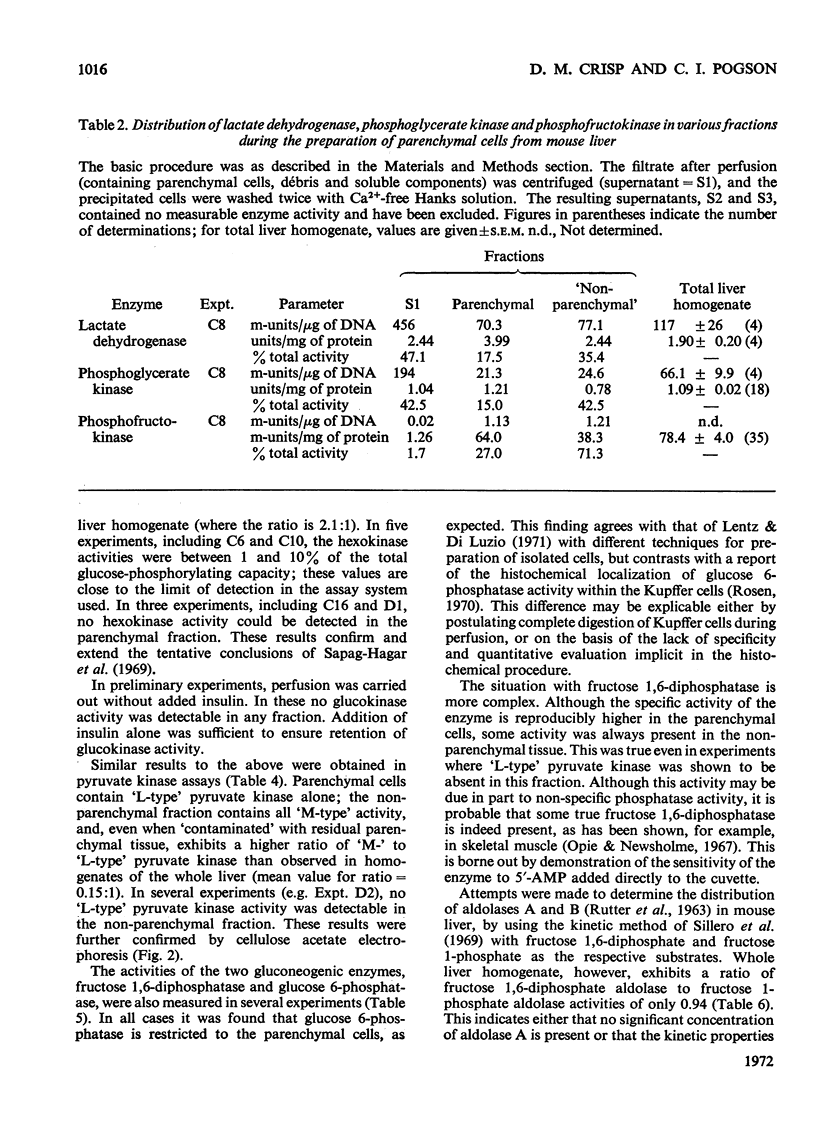
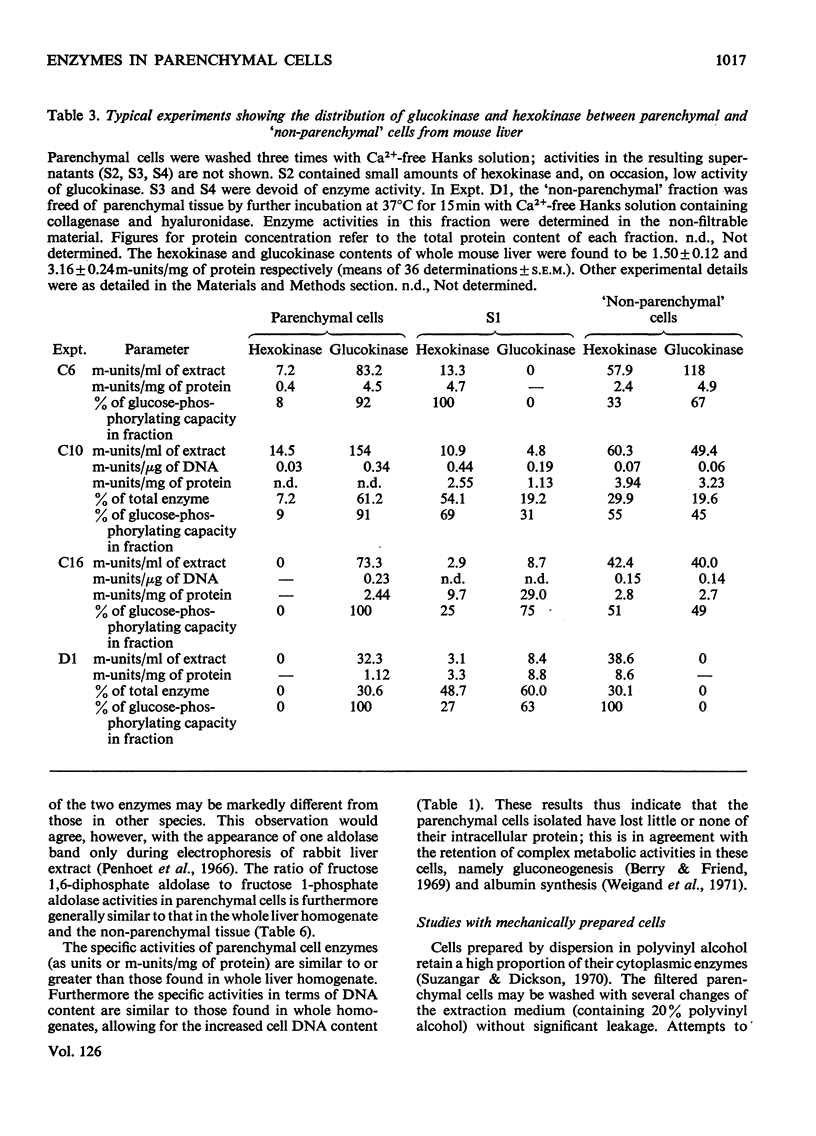
1. Parenchymal cells have been prepared from mouse liver by enzymic and mechanical means. 2. The dry weights, protein and DNA contents of these cells have been determined. 3. Mouse liver `M-' and `L-type' pyruvate kinases have been prepared free of contamination with each other; their kinetic properties have been examined and a method has been developed for their assay in total liver homogenates. 4. Recoveries of phosphoglycerate kinase, lactate dehydrogenase and phosphofructokinase in enzymically prepared cells indicate that little, if any, cytoplasmic protein is lost during preparation. 5. Parenchymal cells exhibit a very substantial increase in the activity ratio of glucokinase to hexokinase over that in total liver homogenate; in three out of eight experiments, hexokinase activity was undetectable. 6. `L-type' pyruvate kinase alone occurs in the parenchymal cell. Non-parenchymal cells are characterized by the presence of `M-type' activity only. 7. Parenchymal cells contain both glucose 6-phosphatase and fructose 1,6-diphosphatase. The non-parenchymal fraction appears to contain fructose 1,6-diphosphatase, but is devoid of glucose 6-phosphatase. 8. No aldolase A was detectable in the whole liver. Aldolase B occurs in both parenchymal and non-parenchymal tissue. 9. Parenchymal cells prepared by mechanical disruption of mouse liver with 20% polyvinyl alcohol exhibit a similar enzyme profile to those prepared enzymically. 10. The methodology involved in the preparation of isolated liver cells is discussed. The importance of the measurement of several parameters as criteria for establishing the viability of parenchymal cells is stressed. 11. The metabolic implications of the results in the present study are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
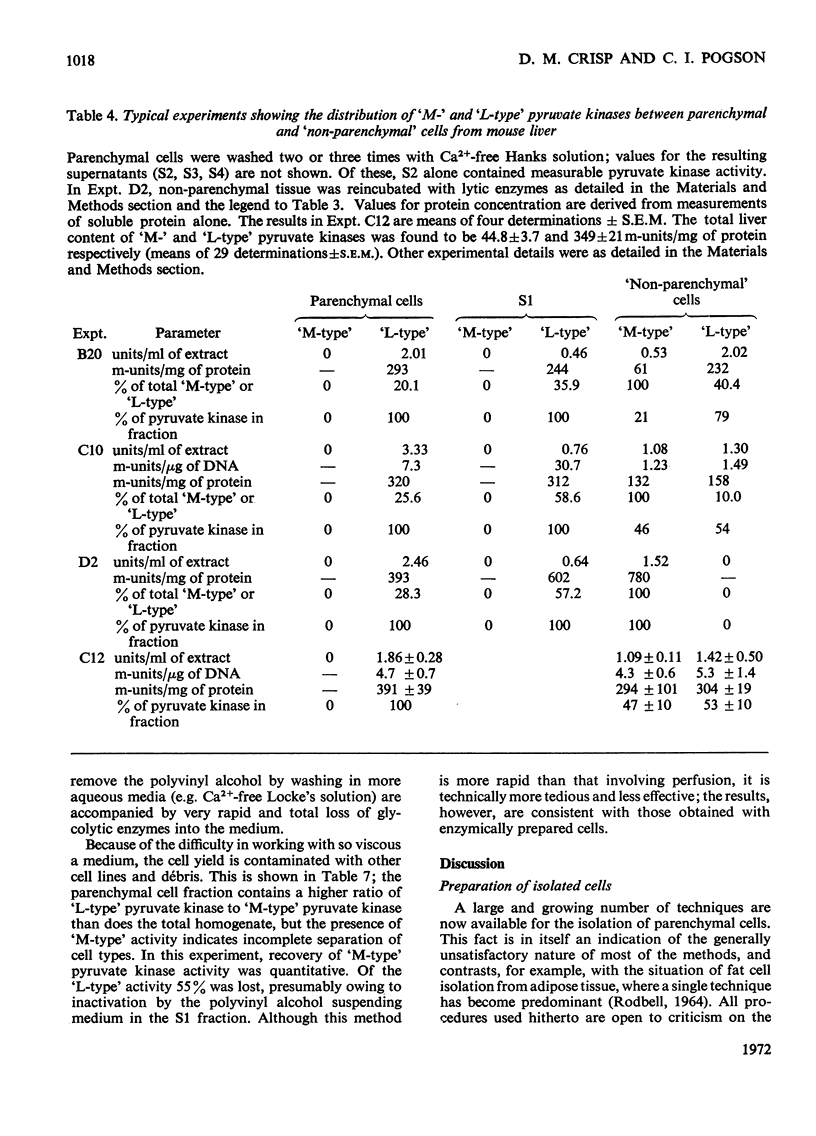
- ARION W. J., NORDLIE R. C. LIVER MICROSOMAL GLUCOSE 6-PHOSPHATASE, INORGANIC PYROPHOSPHATASE, AND PYROPHOSPHATE-GLUCOSE PHOSPHOTRANSFERASE. II. KINETIC STUDIES. J Biol Chem. 1964 Sep;239:2752–2757. [PubMed] [Google Scholar]
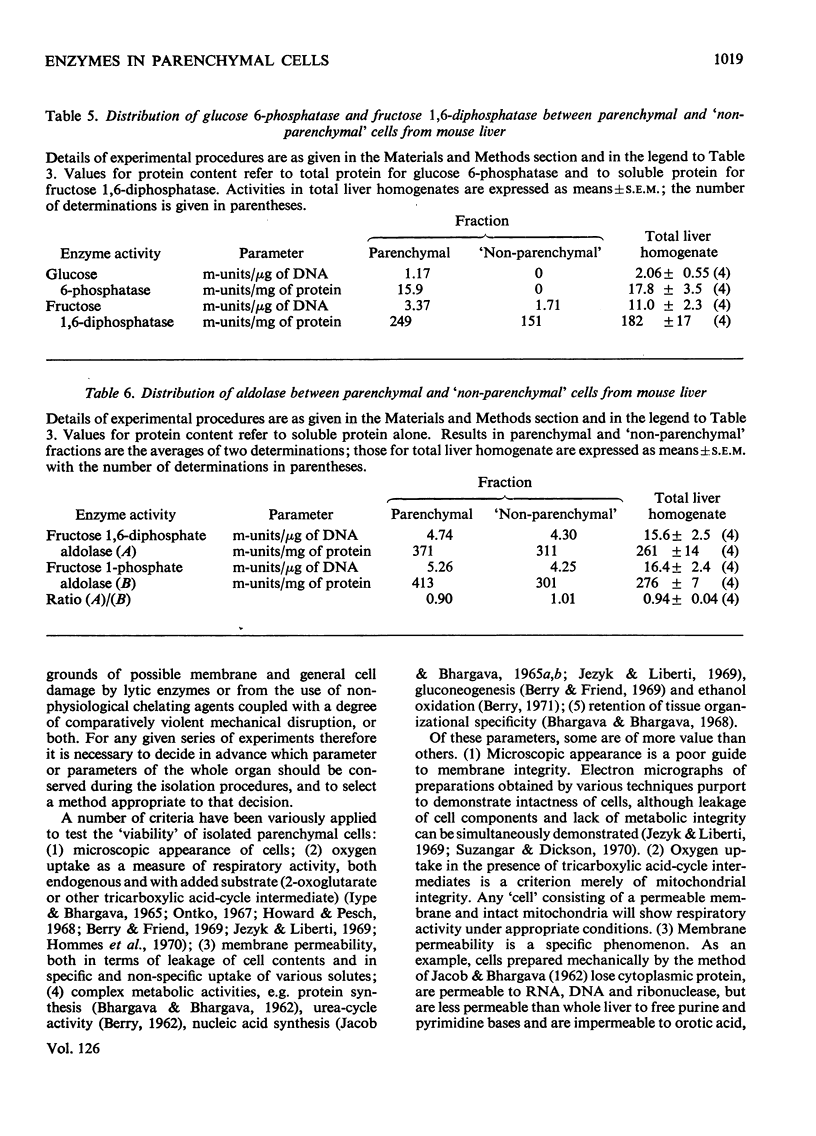
- Achs M. J., Anderson J. H., Garfinkel D. Gluconeogenesis in rat liver cytosol. I. Computer analysis of experimental data. Comput Biomed Res. 1971 Apr;4(1):65–106. doi: 10.1016/0010-4809(71)90047-4. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Randle P. J. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem J. 1970 Aug;119(1):5–15. doi: 10.1042/bj1190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHARGAVA K., BHARGAVA P. M. The incorporation of labelled amino acids into the proteins of liver cells in suspension. Life Sci. 1962 Sep;1:477–482. doi: 10.1016/0024-3205(62)90056-5. [DOI] [PubMed] [Google Scholar]
- BRANSTER M. V., MORTON R. K. Isolation of intact liver cells. Nature. 1957 Dec 7;180(4597):1283–1284. doi: 10.1038/1801283a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Oliver I. T. Ketohexokinase, isoenzymes of glucokinase and glycogen synthesis from hexoses in neonatal rat liver. Biochem J. 1964 Feb;90(2):261–268. doi: 10.1042/bj0900261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthillier G., Colobert L., Richard M., Got R. Glucokinases du foie de rat. Purification et propriétés des formes particulées. Biochim Biophys Acta. 1970 Apr 22;206(1):1–16. doi: 10.1016/0005-2744(70)90076-8. [DOI] [PubMed] [Google Scholar]
- Berthillier G., Got R. Glucokinase microsomique du foie de rat. Un enzyme régulateur de la glycolyse? FEBS Lett. 1970 Jun 1;8(3):122–124. doi: 10.1016/0014-5793(70)80242-3. [DOI] [PubMed] [Google Scholar]
- Bhargava K., Bhargava P. M. In vivo tissue-specific recognition by hepatic cells. Exp Cell Res. 1968 Jun;50(3):515–523. doi: 10.1016/0014-4827(68)90415-1. [DOI] [PubMed] [Google Scholar]
- CAHILL G. F., Jr, ASHMORE J., RENOLD A. E., HASTINGS A. B. Blood glucose and the liver. Am J Med. 1959 Feb;26(2):264–282. doi: 10.1016/0002-9343(59)90316-x. [DOI] [PubMed] [Google Scholar]
- Carminatti H., Jiménez de Asúa L., Recondo E., Passeron S., Rozengurt E. Some kinetic properties of liver pyruvate kinase (type L). J Biol Chem. 1968 Jun 10;243(11):3051–3056. [PubMed] [Google Scholar]
- DIPIETRO D. L., SHARMA C., WEINHOUSE S. Studies on glucose phosphorylation in rat liver. Biochemistry. 1962 May 25;1:455–462. doi: 10.1021/bi00909a014. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Epstein C. J. The incorporation of [3H]leucine into protein by tetraphenylboron- and citrate-dispersed rat liver parenchymal cells. Biochim Biophys Acta. 1967 May 30;138(3):622–624. doi: 10.1016/0005-2787(67)90564-3. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Andersson M. Regulation of the pyruvate kinase of an established rat liver cell line (RLC) in culture by insulin, glucose and serum. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1211–1218. doi: 10.1016/s0006-291x(71)80001-3. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Casanello D. Metabolism of rat liver cells cultured in suspension: insulin and glucagon effects on glycogen level. Biochem Biophys Res Commun. 1968 Nov 25;33(4):584–589. doi: 10.1016/0006-291x(68)90335-5. [DOI] [PubMed] [Google Scholar]
- González C., Ureta T., Sánchez R., Niemeyer H. Multiple molecular forms of ATP:hexose 6-phosphotransferase from rat liver. Biochem Biophys Res Commun. 1964 Jul 1;16(4):347–352. doi: 10.1016/0006-291x(64)90038-5. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- HOSKINS J. M., MEYNELL G. G., SANDERS F. K. A comparison of methods for estimating the viable count of a suspension of tumour cells. Exp Cell Res. 1956 Aug;11(2):297–305. doi: 10.1016/0014-4827(56)90105-7. [DOI] [PubMed] [Google Scholar]
- Heath D. F., Threlfall C. J. The interaction of glycolysis, gluconeogenesis and the tricarboxylic acid cycle in rat liver in vivo. Biochem J. 1968 Nov;110(2):337–362. doi: 10.1042/bj1100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes F. A., Draisma M. I., Molenaar I. Preparation and some properties of isolated rat liver cells. Biochim Biophys Acta. 1970 Nov 24;222(2):361–371. doi: 10.1016/0304-4165(70)90125-x. [DOI] [PubMed] [Google Scholar]
- Howard R. B., Christensen A. K., Gibbs F. A., Pesch L. A. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967 Dec;35(3):675–684. doi: 10.1083/jcb.35.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. B., Pesch L. A. Respiratory activity of intact, isolated parenchymal cells from rat liver. J Biol Chem. 1968 Jun 10;243(11):3105–3109. [PubMed] [Google Scholar]
- IYPE P. T., BHARGAVA P. M. THE RESPIRATION OF ISOLATED RAT-HEPATIC CELLS IN SUSPENSION. Biochem J. 1965 Jan;94:284–288. doi: 10.1042/bj0940284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibsen K. H., Schiller K. W., Haas T. A. Interconvertible kinetic and physical forms of human erythrocyte pyruvate kinase. J Biol Chem. 1971 Mar 10;246(5):1233–1240. [PubMed] [Google Scholar]
- JACOB S. T., BHARGAVA P. M. A new method for the preparation of liver cell suspensions. Exp Cell Res. 1962 Sep;27:453–467. doi: 10.1016/0014-4827(62)90011-3. [DOI] [PubMed] [Google Scholar]
- JACOB S. T., BHARGAVA P. M. METABOLISM OF TISSUE CELLS IN SUSPENSION. SYNTHESIS OF RIBONUCLEIC ACID BY LEIVER CELLS IN SUSPENSION. Biochem J. 1965 May;95:568–576. doi: 10.1042/bj0950568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T., Bhargava P. M. Effect of chloramphenicol on ribonucleic acid synthesis in liver cells in suspension. Biochem J. 1965 Oct;97(1):67–73. doi: 10.1042/bj0970067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezyk P. F., Liberti J. P. Metabolic activities of mechanically and enzymatically prepared rat liver cells. Arch Biochem Biophys. 1969 Nov;134(2):442–449. doi: 10.1016/0003-9861(69)90304-x. [DOI] [PubMed] [Google Scholar]
- Jiménez de Asúa L., Rozengurt E., Devalle J. J., Carminatti H. Some kinetic differences between the M isoenzymes of pyruvate kinase from liver and muscle. Biochim Biophys Acta. 1971 May 12;235(2):326–334. doi: 10.1016/0005-2744(71)90211-7. [DOI] [PubMed] [Google Scholar]
- KATZEN H. M., SODERMAN D. D., NITOWSKY H. M. KINETIC AND ELECTROPHORETIC EVIDENCE FOR MULTIPLE FORMS OF GLUCOSE-ATP PHOSPHOTRANSFERASE ACTIVITY FROM HUMAN CELL CULTURES AND RAT LIVER. Biochem Biophys Res Commun. 1965 Apr 23;19:377–382. doi: 10.1016/0006-291x(65)90472-9. [DOI] [PubMed] [Google Scholar]
- Krietsch W. K., Bücher T. 3-phosphoglycerate kinase from rabbit sceletal muscle and yeast. Eur J Biochem. 1970 Dec;17(3):568–580. doi: 10.1111/j.1432-1033.1970.tb01202.x. [DOI] [PubMed] [Google Scholar]
- LAWS J. O., STICKLAND L. H. The adhesion of liver cells. Exp Cell Res. 1961 Aug;24:240–254. doi: 10.1016/0014-4827(61)90426-8. [DOI] [PubMed] [Google Scholar]
- LING K. H., MARCUS F., LARDY H. A. PURIFICATION AND SOME PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHOFRUCTOKINASE. J Biol Chem. 1965 May;240:1893–1899. [PubMed] [Google Scholar]
- Lentz P. E., Di Luzio N. R. Biochemical characterization of Kupffer and parenchymal cells isolated from rat liver. Exp Cell Res. 1971 Jul;67(1):17–26. doi: 10.1016/0014-4827(71)90616-1. [DOI] [PubMed] [Google Scholar]
- Llorente P., Marco R., Sols A. Regulation of liver pyruvate kinase and the phosphoenolpyruvate crossroads. Eur J Biochem. 1970 Mar 1;13(1):45–54. doi: 10.1111/j.1432-1033.1970.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Murthy L., Petering H. G. A comparison of some biochemical properties of dissociated liver cells and of slices. Proc Soc Exp Biol Med. 1969 Dec;132(3):931–935. doi: 10.3181/00379727-132-34340. [DOI] [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontko J. A. Chylomicron, free fatty acid and ketone body metabolism of isolated liver cells and liver homogenates. Biochim Biophys Acta. 1967 Feb 14;137(1):13–22. doi: 10.1016/0005-2760(67)90003-3. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Newsholme E. A. The activities of fructose 1,6-diphosphatase, phosphofructokinase and phosphoenolpyruvate carboxykinase in white muscle and red muscle. Biochem J. 1967 May;103(2):391–399. doi: 10.1042/bj1030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E., Rajkumar T., Rutter W. J. Multiple forms of fructose diphosphate aldolase in mammalian tissues. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1275–1282. doi: 10.1073/pnas.56.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson C. I. Two interconvertible forms of pyruvate kinase in adipose tissue. Biochem Biophys Res Commun. 1968 Feb 15;30(3):297–302. doi: 10.1016/0006-291x(68)90450-6. [DOI] [PubMed] [Google Scholar]
- RODBELL M. LOCALIZATION OF LIPOPROTEIN LIPASE IN FAT CELLS OF RAT ADIPOSE TISSUE. J Biol Chem. 1964 Mar;239:753–755. [PubMed] [Google Scholar]
- Rappaport C., Howze G. B. Dissociation of adult mouse liver by sodium tetraphenylboron, a potassium complexing agent. Proc Soc Exp Biol Med. 1966 Apr;121(4):1010–1016. doi: 10.3181/00379727-121-30951. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Gluconeogenesis in the kidney cortex. Effects of D-malate and amino-oxyacetate. Biochem J. 1970 Feb;116(3):483–491. doi: 10.1042/bj1160483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. I. The ultrastructural localization of the G-6-P hydrolyzing enzyme activity in Kupffer cells. Experientia. 1970 Aug 15;26(8):839–840. doi: 10.1007/BF02114207. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Jiménez de Asúa L., Carminatti H. Some kinetic properties of liver pyruvate kinase (type L). II. Effect of pH on its allosteric behavior. J Biol Chem. 1969 Jun 25;244(12):3142–3147. [PubMed] [Google Scholar]
- SALAS J., SALAS M., VINUELA E., SOLS A. GLUCOKINASE OF RABBIT LIVER. J Biol Chem. 1965 Mar;240:1014–1018. [PubMed] [Google Scholar]
- SHARMA R. M., SHARMA C., DONNELLY A. J., MORRIS H. P., WEINHOUSE S. GLUCOSE-ATP PHOSPHOTRANSFERASES DURING HEPATOCARCINOGENESIS. Cancer Res. 1965 Feb;25:193–199. [PubMed] [Google Scholar]
- Sapag-Hagar M., Marco R., Sols A. Distribution of hexokinase and glucokinase between parenchymal and non-parenchymal cells of rat liver. FEBS Lett. 1969 Apr;3(1):68–71. doi: 10.1016/0014-5793(69)80099-2. [DOI] [PubMed] [Google Scholar]
- Sillero M. A., Sillero A., Sols A. Enzymes involved in fructose metabolism in lir and the glyceraldehyde metabolic crossroads. Eur J Biochem. 1969 Sep;10(2):345–350. doi: 10.1111/j.1432-1033.1969.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sols A., Salas M., Viñuela E. Induced biosynthesis of liver glucokinase. Adv Enzyme Regul. 1964;2:177–188. doi: 10.1016/s0065-2571(64)80012-1. [DOI] [PubMed] [Google Scholar]
- Susor W. A., Rutter W. J. Some distinctive properties of pyruvate kinase purified from rat liver. Biochem Biophys Res Commun. 1968 Jan 11;30(1):14–20. doi: 10.1016/0006-291x(68)90705-5. [DOI] [PubMed] [Google Scholar]
- Suzangar M., Dickson J. A. Biochemical studies on cells isolated from adult rat liver. Exp Cell Res. 1970 Dec;63(2):353–364. doi: 10.1016/0014-4827(70)90223-5. [DOI] [PubMed] [Google Scholar]
- Szepesi B., Avery E. H., Freedland R. A. Role of kidney in gluconeogenesis and amino acid catabolism. Am J Physiol. 1970 Dec;219(6):1627–1631. doi: 10.1152/ajplegacy.1970.219.6.1627. [DOI] [PubMed] [Google Scholar]
- TAKEDA Y., ICHIHARA A., TANIOKA H., INOUE H. THE BIOCHEMISTRY OF ANIMAL CELLS. I. THE EFFECT OF CORTICOTEROIDS ON LEAKAGE OF ENZYMES FROM DISPERSED RAT LIVER CELLS. J Biol Chem. 1964 Oct;239:3590–3596. [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Morimura H., Mori R. Evidence for the presence of two types of pyruvate kinase in rat liver. Biochem Biophys Res Commun. 1965 Oct 8;21(1):55–60. doi: 10.1016/0006-291x(65)90425-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Sue F., Morimura H. Feed-forward activation and feed-back inhibition of pyruvate kinase type L of rat liver. Biochem Biophys Res Commun. 1967 Nov 17;29(3):444–449. doi: 10.1016/0006-291x(67)90477-9. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneziale C. M., Gabrielli F., Lardy H. A. Gluconeogenesis from pyruvate in isolated perfused rat liver. Biochemistry. 1970 Sep 29;9(20):3960–3970. doi: 10.1021/bi00822a014. [DOI] [PubMed] [Google Scholar]
- Veneziale C. M. Gluconeogenesis in the isolated rat liver. Studies with bicarbonate-14C. Biochemistry. 1971 Jul 6;10(14):2793–2798. doi: 10.1021/bi00790a022. [DOI] [PubMed] [Google Scholar]
- WILSON M. E., STOWELL R. E., YOKOYAMA H. O., TSUBOI K. K. Cytological changes in regenerating mouse liver. Cancer Res. 1953 Jan;13(1):86–92. [PubMed] [Google Scholar]
- Weignand K., Müller M., Urban J., Schreiber G. Intact endoplasmic reticulum and albumin synthesis in rat liver cell suspensions. Exp Cell Res. 1971 Jul;67(1):27–32. doi: 10.1016/0014-4827(71)90617-3. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Jákob A., Scholz R. Energy cost of gluconeogenesis in rat liver. Metabolism. 1971 Jan;20(1):13–26. doi: 10.1016/0026-0495(71)90056-4. [DOI] [PubMed] [Google Scholar]


