Abstract
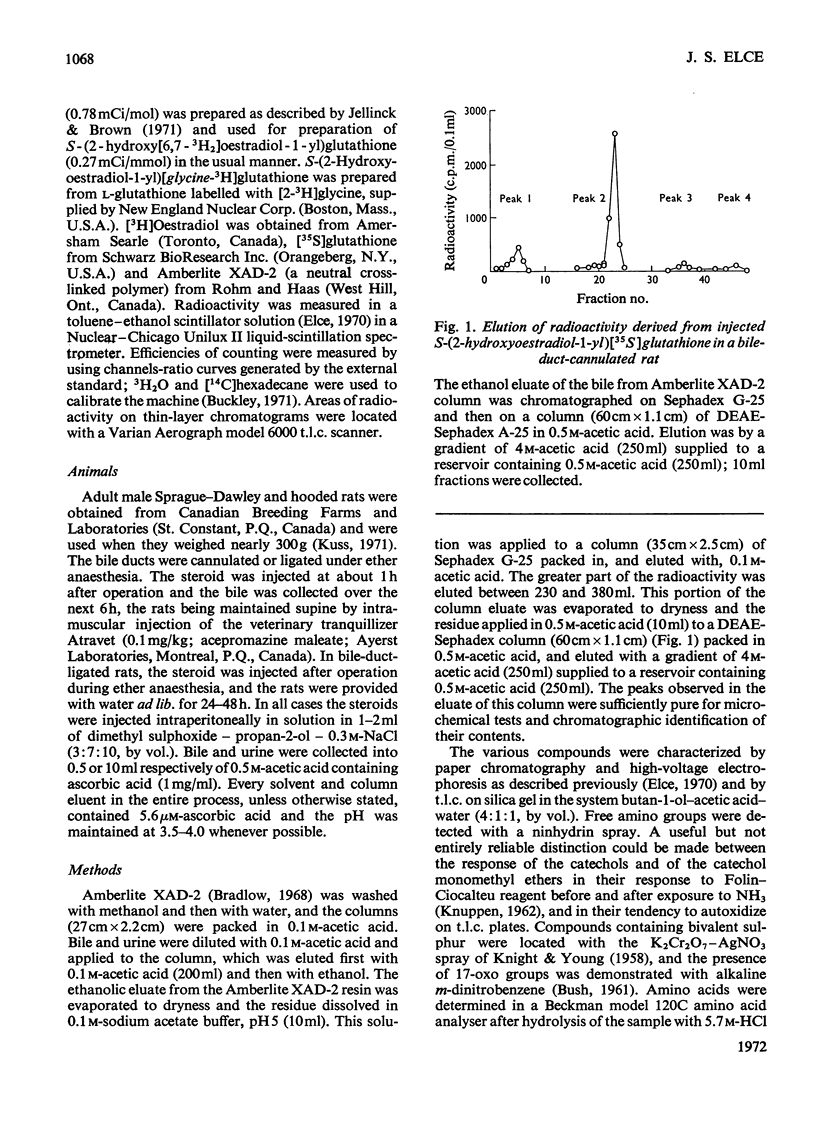
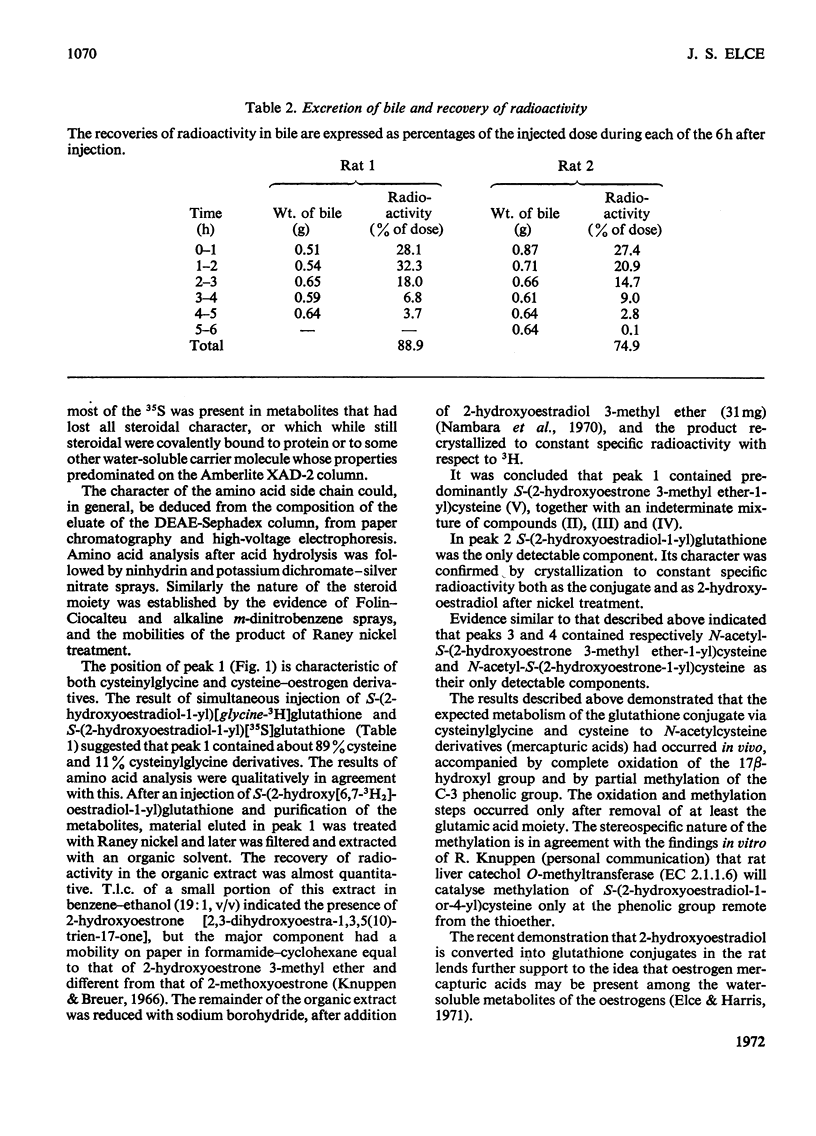
Adult male rats with cannulated or ligated bile ducts were given S-(2-hydroxyoestradiol-1-yl)[35S]glutathione, S-(2-hydroxy[6,7-3H2]oestradiol-1-yl)glutathione or S-(2-hydroxyoestradiol-1-yl)[glycine-3H]glutathione by intraperitoneal injection. The recovery of radioactivity in the bile of bile duct-cannulated rats was 33–86% and in the urine of bile duct-ligated rats was 54–105%. Oestrogen thioether derivatives of glutathione, cysteinylglycine, cysteine and N-acetylcysteine were isolated from bile; only the N-acetylcysteine derivatives could be identified in the urine. The steroid moiety was characterized by microchemical tests before and after treatment with Raney nickel: 2-hydroxyoestradiol-17β was released from the glutathione conjugate, and 2-hydroxyoestrone and 2-hydroxyoestrone 3-methyl ether from the other conjugates. From intact rats the recovery of administered radioactivity was about 15% in the urine and 5% in the faeces over a period of several days and the radioactivity appeared to be largely protein-bound. The results demonstrate that injected oestrogen–glutathione conjugate undergoes conversion into N-acetylcysteine derivatives in vivo. Oestrogen–glutathione conjugates formed in the intact rat may be excreted in an apparently non-steroidal, possibly protein-bound form, which would not be detected by current analytical techniques.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAY H. G., FRANKLIN T. J., JAMES S. P. The formation of mercapturic acids. 2. The possible role of glutathionase. Biochem J. 1959 Apr;71(4):690–696. doi: 10.1042/bj0710690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlow H. L. Extraction of steroid conjugates with a neutral resin. Steroids. 1968 Mar;11(3):265–272. doi: 10.1016/s0039-128x(68)80139-4. [DOI] [PubMed] [Google Scholar]
- Buckley J. P. The use of carbon-14 as a secondary counting standard for sulphur-35. Int J Appl Radiat Isot. 1971 Jan;22(1):41–42. doi: 10.1016/0020-708x(71)90157-8. [DOI] [PubMed] [Google Scholar]
- Elce J. S., Harris J. Conjugation of 2-hydroxyestradiol-17 (1,3,5(10)-estratriene-2,3,17 -triol) with glutathione in the rat. Steroids. 1971 Nov;18(5):583–591. doi: 10.1016/0039-128x(71)90071-7. [DOI] [PubMed] [Google Scholar]
- Elce J. S. Metabolism of a glutathione conjugate of 2-hydroxyoestradiol by rat liver and kidney preparations in vitro. Biochem J. 1970 Mar;116(5):913–917. doi: 10.1042/bj1160913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinck P. H., Brown B. J. A simple enzymatic method for the synthesis of 2-hydroxy[4-14C] estradiol. Steroids. 1971 Jan;17(1):133–140. doi: 10.1016/s0039-128x(71)80120-4. [DOI] [PubMed] [Google Scholar]
- Jellinck P. H., Lewis J., Boston F. Further evidence for the formation of an estrogen-peptide conjugate by rat liver in vitro. Steroids. 1967 Sep;10(3):329–346. doi: 10.1016/0039-128x(67)90058-x. [DOI] [PubMed] [Google Scholar]
- KNIGHT R. H., YOUNG L. Biochemical studies of toxic agents. 11. The occurrence of premercapturic acids. Biochem J. 1958 Sep;70(1):111–119. doi: 10.1042/bj0700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss E. Mikrosomale Oxidation des Ostradiols-17 beta. 2-Hydroxylierung und 1- bzw. 4- Thioätherbildung mit und ohne 17-Hydroxyl-Dehydrogenierung. Hoppe Seylers Z Physiol Chem. 1971 Jun;352(6):817–836. [PubMed] [Google Scholar]


