Abstract
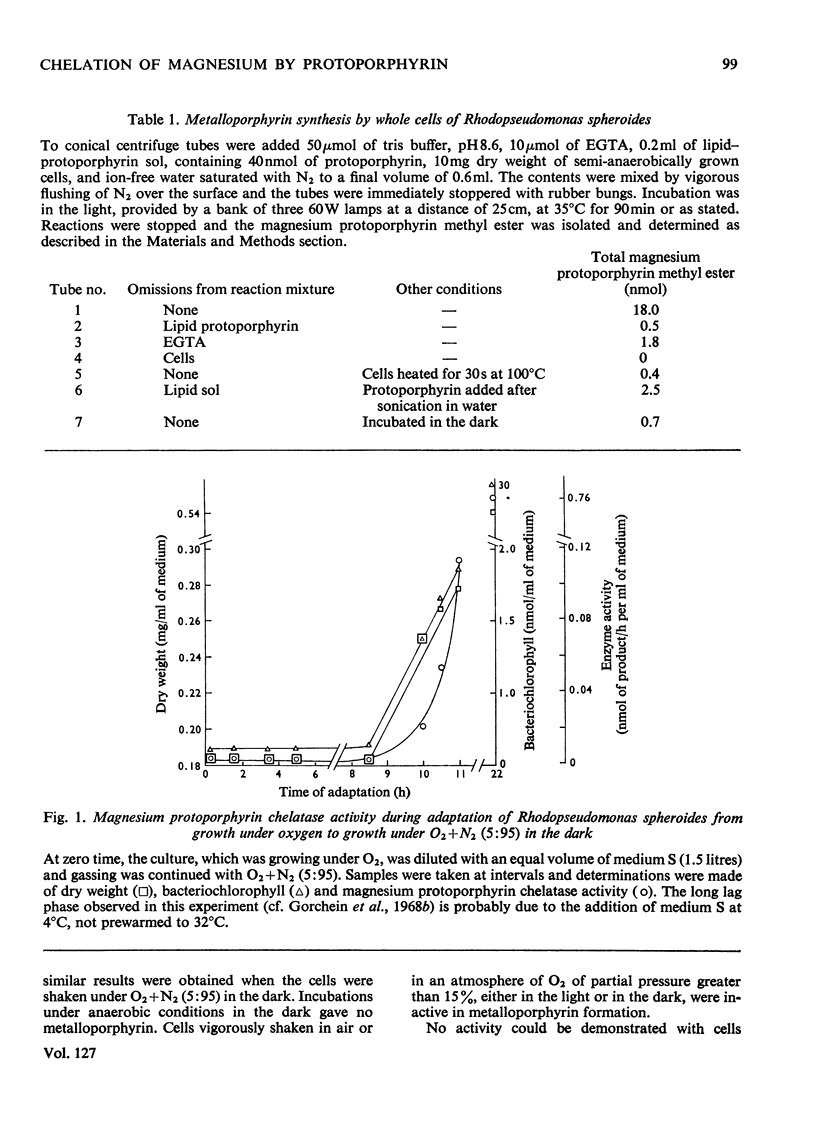
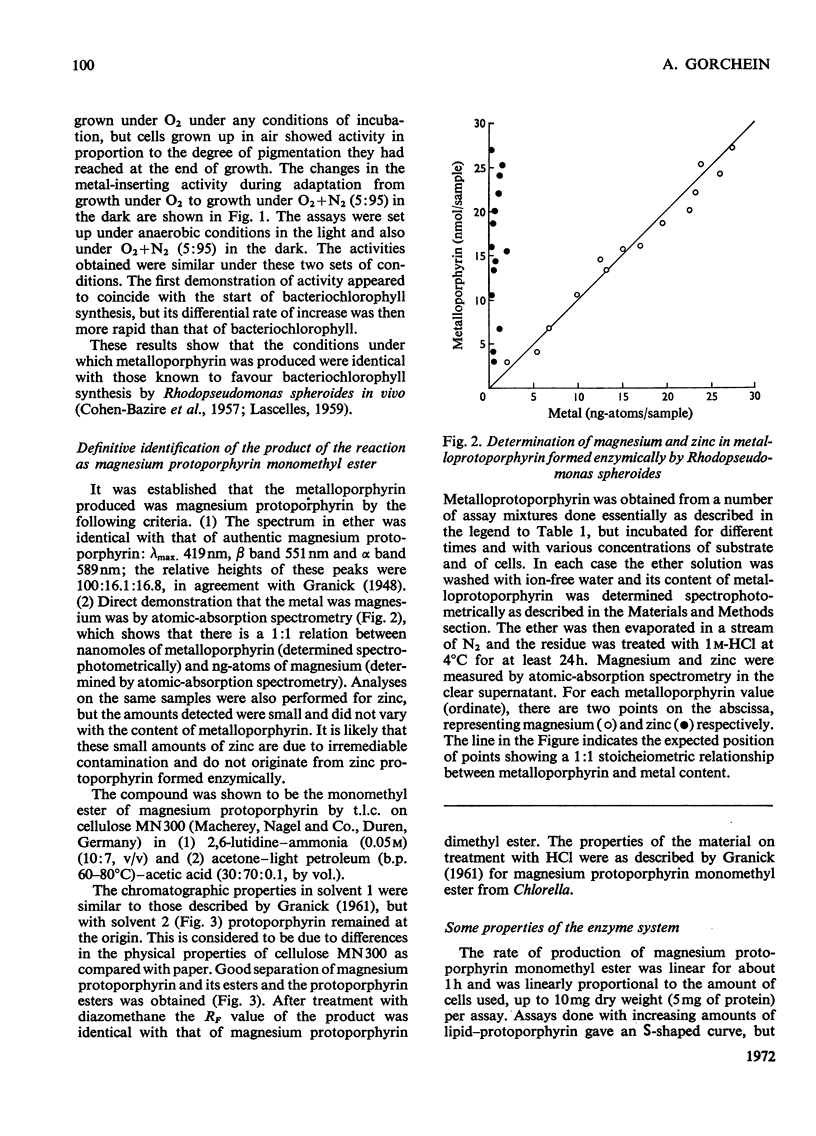
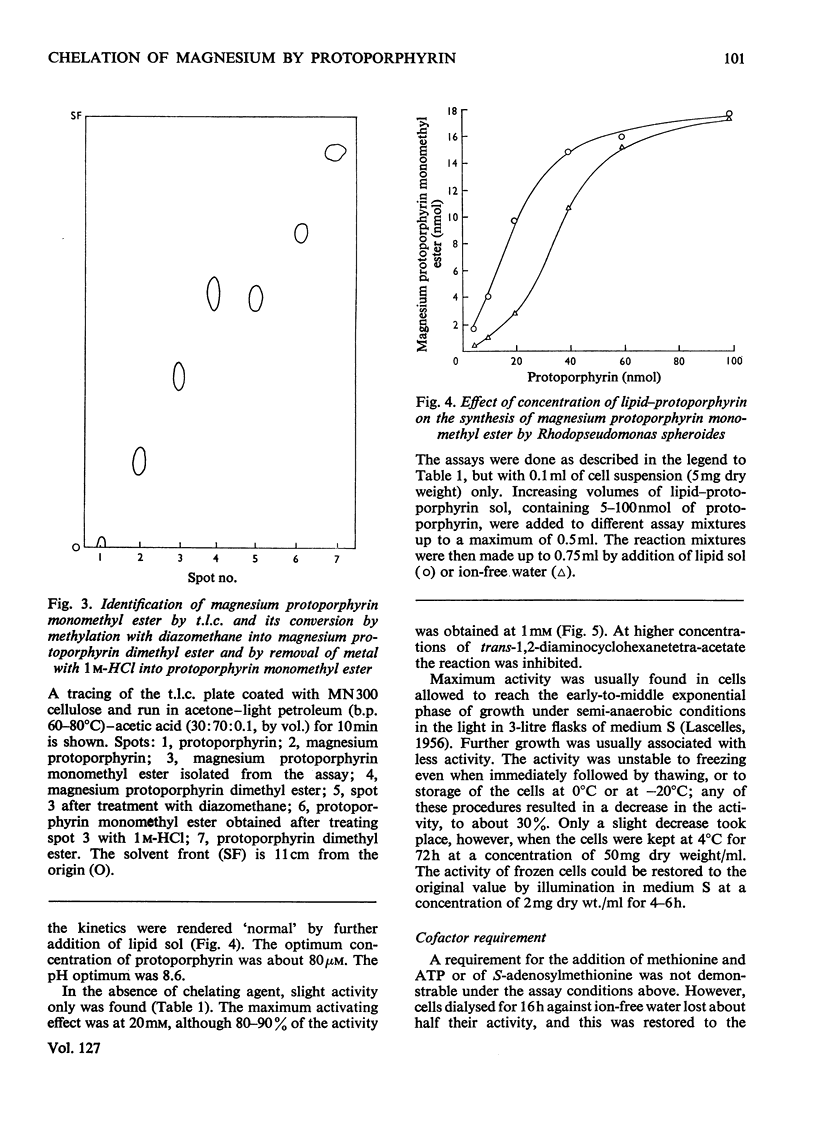
1. Whole cells of Rhodopseudomonas spheroides grown under semi-anaerobic conditions in the light incorporated magnesium into exogenous protoporphyrin when incubated with EDTA or the related chelators EGTA, N-(2-hydroxyethyl)-ethylenediamine-NN′N′- triacetate and trans-1,2-diaminocyclohexanetetra-acetate. 2. The reaction was demonstrated under anaerobic conditions in the light or at low oxygen partial pressure in the dark. Partial pressures of oxygen greater than 15% inhibited the reaction. 3. Cells grown under pure oxygen were completely inactive, but on adaptation to growth under low oxygen partial pressure (O2+N2, 5:95) the development of activity paralleled the synthesis of bacteriochlorophyll. 4. The reaction with normal cells did not require protein synthesis, but cells that had lost their activity by being illuminated in Mg2+-deficient medium did not recover it in the absence of protein synthesis. 5. The product of the reaction was magnesium protoporphyrin monomethyl ester. 6. Evidence is presented that insertion of magnesium is obligatorily coupled with methylation and it is concluded that the reaction is dependent on a multienzyme complex.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUM S. J., BURNHAM B. F., PLANE R. A. STUDIES ON THE BIOSYNTHESIS OF CHLOROPHYLL: CHEMICAL INCORPORATION OF MAGNESIUM INTO PORPHYRINS. Proc Natl Acad Sci U S A. 1964 Dec;52:1439–1442. doi: 10.1073/pnas.52.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BULL M. J., LASCELLES J. The association of protein synthesis with formation of pigments in some photosynthetic bacteria. Biochem J. 1963 Apr;87:15–28. doi: 10.1042/bj0870015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Ebbon J. G., Tait G. H. Studies on S-adenosylmethionine-magnesium protoporphyrin methyltransferase in Euglena gracilis strain Z. Biochem J. 1969 Feb;111(4):573–582. doi: 10.1042/bj1110573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Magnesium protoporphyrin monoester and protoporphyrin monomethyl ester in chlorophyll biosynthesis. J Biol Chem. 1961 Apr;236:1168–1172. [PubMed] [Google Scholar]
- Gorchein A., Neuberger A., Tait G. H. Adaptation of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Aug 13;171(1022):111–125. doi: 10.1098/rspb.1968.0060. [DOI] [PubMed] [Google Scholar]
- Gorchein A., Neuberger A., Tait G. H. The isolation and characterization of subcellular fractions from pigmented and unpigmented cells of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Jul 2;170(1020):229–246. doi: 10.1098/rspb.1968.0035. [DOI] [PubMed] [Google Scholar]
- JONES O. T. The production of magnesium protoporphyrin monomethyl ester by Rhodopseudomonas spheroides. Biochem J. 1963 Mar;86:429–432. doi: 10.1042/bj0860429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Ferrochelatase of Rhodopseudomonas spheroides. Biochem J. 1970 Sep;119(3):453–462. doi: 10.1042/bj1190453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZEN H. M., BUCHANAN J. M. ENZYMATIC SYNTHESIS OF THE METHYL GROUP OF METHIONINE. 8. REPRESSION-DEREPRESSION, PURIFICATION, AND PROPERTIES OF 5,10-METHYLENETETRAHYDROFOLATE REDUCTASE FROM ESCHERICHIA COLI. J Biol Chem. 1965 Feb;240:825–835. [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J., Altschuler T. Mutant strains of Rhodopseudomonas spheroides lacking delta-aminolevulinate synthase: growth, heme, and bacteriochlorophyll synthesis. J Bacteriol. 1969 May;98(2):721–727. doi: 10.1128/jb.98.2.721-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J. The accumulation of bacteriochlorophyll precursors by mutant and wild-type strains of Rhodopseudomonas spheroides. Biochem J. 1966 Jul;100(1):175–183. doi: 10.1042/bj1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- MORELL D. B., BARRETT J., CLEZY P. S. The prosthetic group of cytochrome oxidase. 1. Purification as porphyrin alpha and conversion into haemin alpha. Biochem J. 1961 Apr;78:793–797. doi: 10.1042/bj0780793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD S. H. Activation of methionine for transmethylation. VI. Enzyme-bound tripolyphosphate as an intermediate in the reaction catalyzed by the methionine-activating enzyme of Baker's yeast. J Biol Chem. 1963 Jun;238:2156–2163. [PubMed] [Google Scholar]
- Mazanowska A. M., Neuberger A., Tait G. H. Effect of lipids and organic solvents on the enzymic formation of zinc protoporphyrin and haem. Biochem J. 1966 Jan;98(1):117–127. doi: 10.1042/bj0980117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Neuberger A., Tait G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 5. Zinc-protoporphyrin chelatase. Biochem J. 1964 Mar;90(3):607–616. doi: 10.1042/bj0900607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., LASCELLES J. HAEMOPROTEINS AND HAEM SYNTHESIS IN FACULTATIVE PHOTOSYNTHETIC AND DENITRIFYING BACTERIA. Biochem J. 1965 Jan;94:120–126. doi: 10.1042/bj0940120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. Observations on the relationship between the formation of photopigments and the synthesis of protein in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:599–605. doi: 10.1099/00221287-28-4-599. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. The kinetics of the synthesis of photopigments in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:607–616. doi: 10.1099/00221287-28-4-607. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]


