Abstract
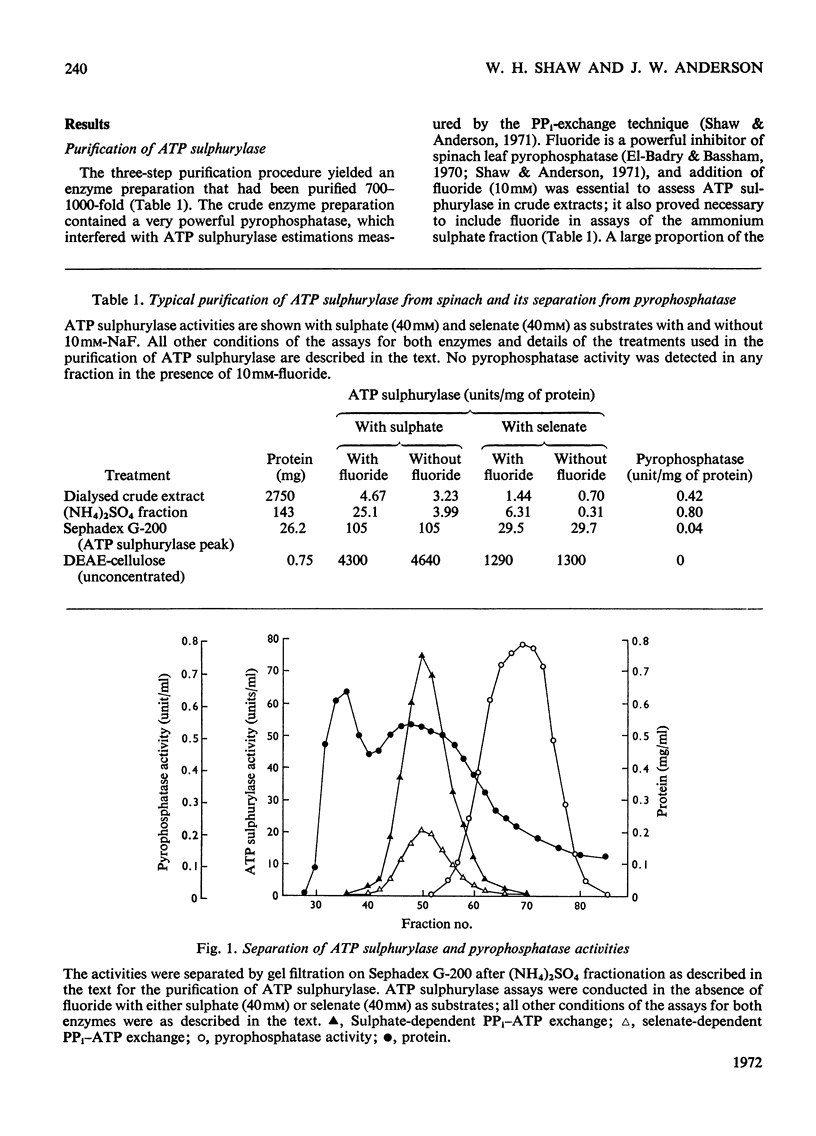
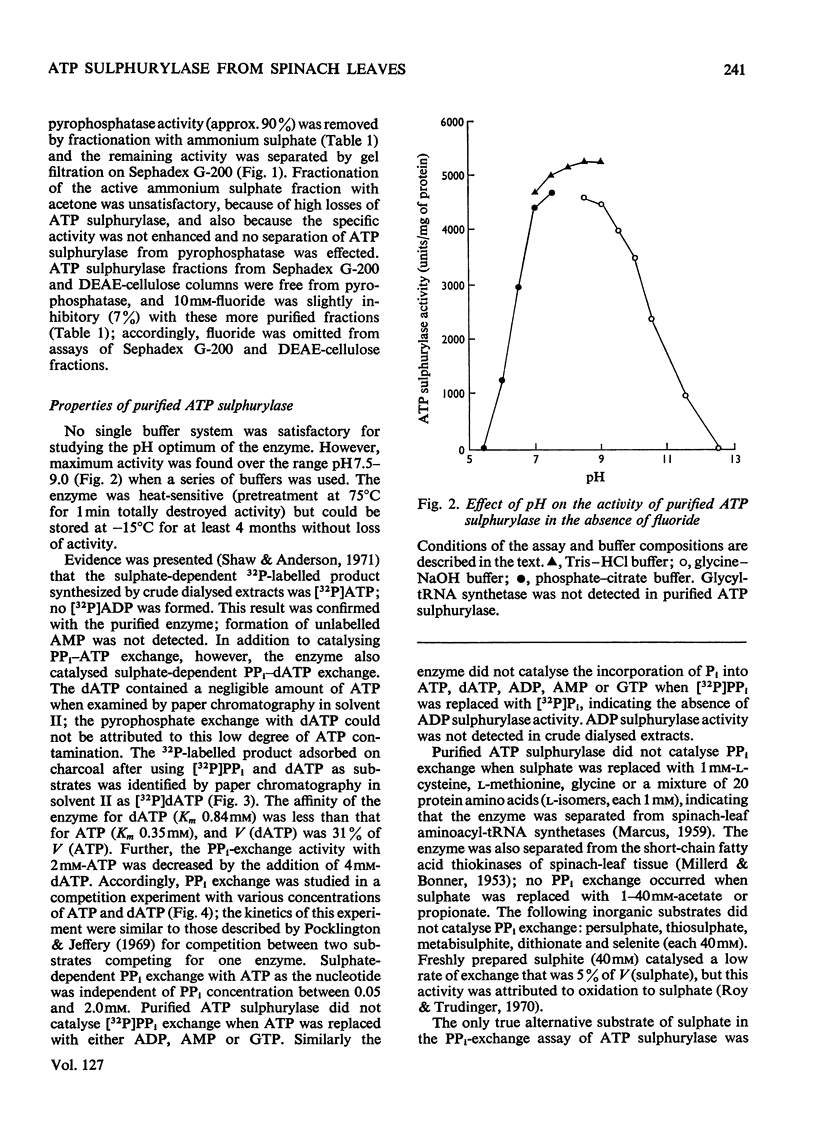
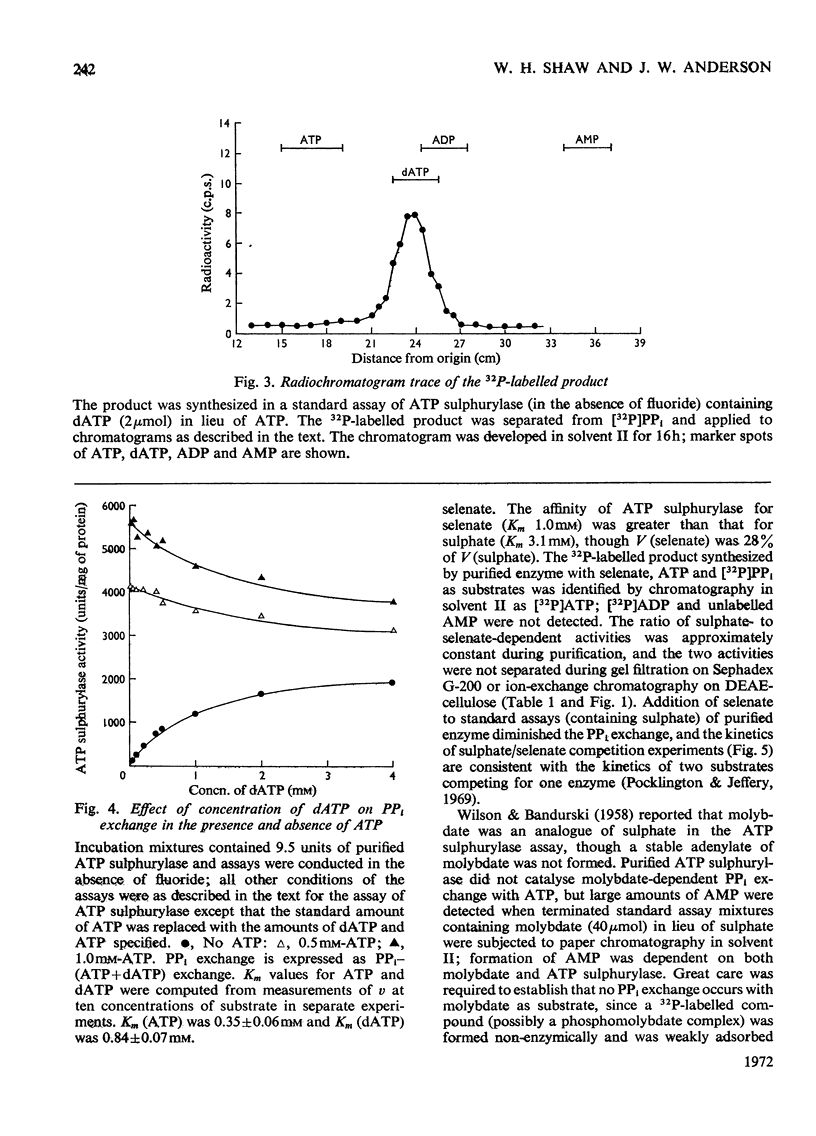
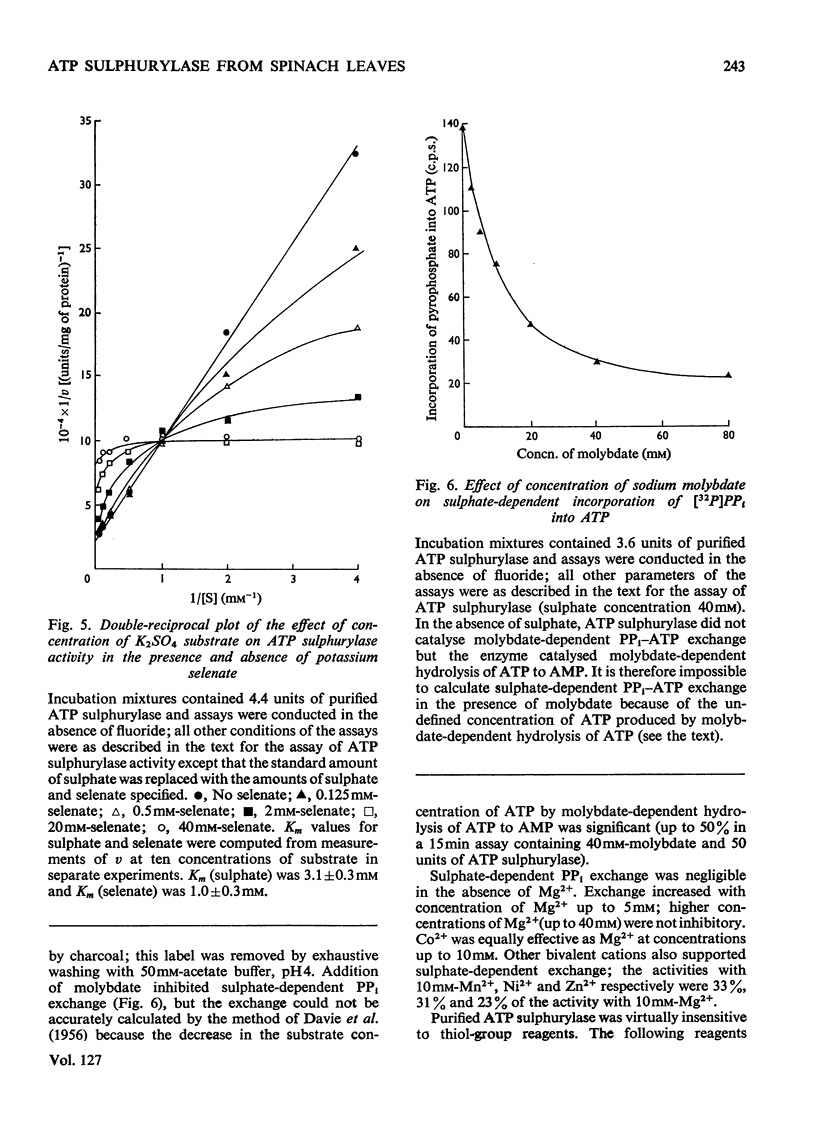
1. ATP sulphurylase was purified up to 1000-fold from spinach leaf tissue. Activity was measured by sulphate-dependent [32P]PPi–ATP exchange. The enzyme was separated from Mg2+-requiring alkaline pyrophosphatase (which interferes with the PPi–ATP-exchange assay) and from other PPi–ATP-exchange activities. No ADP sulphurylase activity was detected. 2. Sulphate was the only form of inorganic sulphur that catalysed PPi–ATP exchange; Km (sulphate) was 3.1mm, Km (ATP) was 0.35mm and the pH optimum was 7.5–9.0. The enzyme was insensitive to thiol-group reagents and required either Mg2+ or Co2+ for activity. 3. The enzyme catalysed [32P]PPi–dATP exchange; Km (dATP) was 0.84mm and V (dATP) was 30% of V (ATP). Competition between ATP and dATP was demonstrated. 4. Selenate catalysed [32P]PPi–ATP exchange and competed with sulphate; Km (selenate) was 1.0mm and V (selenate) was 30% of V (sulphate). No AMP was formed with selenate as substrate. Molybdate did not catalyse PPi–ATP exchange, but AMP was formed. 5. Synthesis of adenosine 5′-[35S]sulphatophosphate was demonstrated by coupling purified ATP sulphurylase and Mg2+-dependent alkaline pyrophosphatase (also prepared from spinach) with [35S]sulphate and ATP as substrates; adenosine 5′-sulphatophosphate was not synthesized in the absence of pyrophosphatase. Some parameters of the coupled system are reported.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Johnson R. E. ATP Sulfurylase Activity in the Soybean [Glycine max (L.) Merr.]. Plant Physiol. 1968 Dec;43(12):2041–2044. doi: 10.1104/pp.43.12.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C. A., Rinne R. W. Influence of age and sulfur metabolism on ATP sulfurylase activity in the soybean and a survey of selected species. Plant Physiol. 1969 Sep;44(9):1241–1246. doi: 10.1104/pp.44.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Fowden L. Properties and substrate specificities of the phenylalanyl-transfer-ribonucleic acid synthetases of Aesculus species. Biochem J. 1970 Oct;119(4):677–690. doi: 10.1042/bj1190677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balharry G. J., Nicholas D. J. ATP-sulphurylase in spinach leaves. Biochim Biophys Acta. 1970 Dec 16;220(3):513–524. doi: 10.1016/0005-2744(70)90282-2. [DOI] [PubMed] [Google Scholar]
- Balharry G. J., Nicholas D. J. New assay for ATP-sulphurylase using the luciferin-luciferase method. Anal Biochem. 1971 Mar;40(1):1–17. doi: 10.1016/0003-2697(71)90078-9. [DOI] [PubMed] [Google Scholar]
- DAVIE E. W., KONINGSBERGER V. V., LIPMANN F. The isolation of a tryptophan-activating enzyme from pancreas. Arch Biochem Biophys. 1956 Nov;65(1):21–38. doi: 10.1016/0003-9861(56)90173-4. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. The biuret reaction: changes in the ultraviolet absorption spectra and its application to the determination of peptide bonds. Anal Biochem. 1962 Jan;3:40–48. doi: 10.1016/0003-2697(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Ellis P. An act of love ... or an admission of failure? Euthanasia. Nurs Times. 1992 Sep 9;88(37):34–35. [PubMed] [Google Scholar]
- Grunberg-Manago M., Del Campillo-Campbell A., Dondon L., Michelson A. M. ADP-sulfurylase de levure catalysant un échange entre l'orthophosphate et le phosphate terminal des nucleosides diphosphates. Biochim Biophys Acta. 1966 Jul 20;123(1):1–16. [PubMed] [Google Scholar]
- Leggett J. E., Epstein E. Kinetics of Sulfate Absorption by Barley Roots. Plant Physiol. 1956 May;31(3):222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A. S., Wolf G. Purification and properties of the enzyme ATP-sulfurylase and its relation to vitamin A. Biochim Biophys Acta. 1969 Apr 22;178(2):262–282. doi: 10.1016/0005-2744(69)90395-7. [DOI] [PubMed] [Google Scholar]
- MARCUS A. Amino acid dependent exchange between pyrophosphate and adenosine triphosphate in spinach preparations. J Biol Chem. 1959 May;234(5):1238–1240. [PubMed] [Google Scholar]
- MILLERD A., BONNER J. Acetate activation and acetoacetate formation in plant systems. Arch Biochem Biophys. 1954 Apr;49(2):343–355. doi: 10.1016/0003-9861(54)90204-0. [DOI] [PubMed] [Google Scholar]
- NAGANNA B., VENUGOPAL B., SRIPATHI C. E. Occurrence of alkaline pyrophosphatase in vegetable tissues. Biochem J. 1955 Jun;60(2):224–225. doi: 10.1042/bj0600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikkar K. R., Bachhawat B. K. Purification and properties of ATP-sulphate adenylyltransferase from liver. Biochim Biophys Acta. 1968 Mar 25;151(3):725–727. doi: 10.1016/0005-2744(68)90032-6. [DOI] [PubMed] [Google Scholar]
- Peterson P. J., Fowden L. Purification, properties and comparative specificities of the enzyme prolyl-transfer ribonucleic acid synthetase from Phaseolus aureus and Polygonatum multiflorum. Biochem J. 1965 Oct;97(1):112–124. doi: 10.1042/bj0970112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocklington T., Jeffery J. Competition of two substrates for a single enzyme. A simple kinetic theorem exemplified by a hydroxy steroid dehydrogenase reaction. Biochem J. 1969 Apr;112(3):331–334. doi: 10.1042/bj1120331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Enzymatic synthesis of adenosine-5'-phosphosulfate. J Biol Chem. 1958 Sep;233(3):686–690. [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Assay of adenosine 5-triphosphate sulfurylase by pyrophosphate exchange. Plant Physiol. 1971 Jan;47(1):114–118. doi: 10.1104/pp.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- el-Badry A. M., Bassham J. A. Chloroplast inorganic pyrophosphatase. Biochim Biophys Acta. 1970 Mar 3;197(2):308–316. doi: 10.1016/0005-2728(70)90042-3. [DOI] [PubMed] [Google Scholar]


