ABSTRACT
In the search for novel treatment strategies for alcohol use disorder (AUD), glucagon‐like peptide‐1 (GLP‐1) receptor agonists (GLP‐1RAs) approved for treating Type 2 diabetes and obesity have caught much attention. GLP‐1 is a naturally occurring peptide produced in the small intestines and the brain, regulating plasma glucose levels and satiety. This focused review will report on the preclinical studies, case stories, register‐based cohort studies, brain‐imaging data and secondary analysis of clinical data supporting the role of GLP‐1RAs as a novel treatment of AUD. Several clinical trials are ongoing, examining the potential effects of the GLP‐1RA semaglutide in AUD.
Keywords: alcohol use disorder, AUD, fMRI, GLP‐1, glucagon‐like peptide‐1
Plain English Summary.
This review presents the existing data from animal studies in rodents and non‐human primates, clinical trials, register studies and social media investigations, all investigating the potential of a class of diabetes and weight‐loss medications—glucagon‐like peptide‐1 (GLP‐1) receptor agonists—as a novel and very much‐needed treatment for alcohol use disorder.
1. Alcohol Use Disorder (AUD)
AUD is a chronic relapsing brain disorder characterised by loss of control of alcohol intake, compulsive alcohol behaviour leading to relapse and a negative affective state when not consuming alcohol [1]. Up to 50% of AUD patients experience alcohol withdrawal symptoms such as nausea, tremors, and anxiety, and some need medical assistance for detoxification [2]. Globally, AUD is a tremendous burden, with an estimated 280 million people suffering from this disorder [3]. The treatment gap is wide compared to other mental health disorders [4], and a recent Danish register study reports that the all‐cause 10‐year cumulative mortality rate after a first‐time hospital contact due to an alcohol‐related problem is as high as 29% [5]. In this perspective, AUD is a severe condition with enormous consequences for the individual, relatives, and society [2, 3], and regarded as the most harmful addictive drug when taking harm to both users and others into consideration [6]. Several behavioural and psychological treatments are available in the clinic against AUD and have demonstrated efficacy in clinical trials [2]. Cognitive behavioural therapy (CBT) is among the AUD treatments with the highest level of empirical support [7]. According to the National Institute for Health and Care Excellence (NICE) clinical guidelines, a combination of psychological intervention and pharmacological treatment is recommended in patients with moderate to severe AUD [8]. Four medical treatments have been approved by the European Medicines Agency (EMA), that is, disulfiram, acamprosate, naltrexone and nalmefene, and three medical treatments, that is, disulfiram, acamprosate and naltrexone, are approved by the U.S. Food and Drug Administration (FDA) [9]. According to the NICE guidelines, naltrexone and acamprosate, which have shown anticraving efficacy, are first‐line treatments, whereas disulfiram is listed as a second‐line treatment [8]. The opioid receptor antagonist naltrexone is approved as an oral formulation once daily and as a long‐acting injection formulation [9], whereas acamprosate requires dosing thrice daily [9]. Its chemical structure resembles the structure of gamma‐aminobutyric acid (GABA), and preclinical evidence suggests that the effects of acamprosate in AUD are due to interactions with the neurotransmitters GABA and glutamate, restoring the imbalance of neuronal excitation and inhibition caused by chronic alcohol exposure [10]. Disulfiram inhibits the enzyme aldehyde dehydrogenase, which catalyses alcohol conversion to the toxic metabolite acetaldehyde. The enzymatic inhibition causes the ‘disulfiram‐ethanol’ reaction, that is, nausea, vomiting, headache, facial flushing, hypotension, sweating, palpitations, restlessness, exhaustion, confusion and rarely cardiovascular relapse [9, 11]. This means that the therapeutic effect is mediated through the anticipation of getting a disulfiram‐ethanol reaction [9]. Nalmefene is a mu‐ and delta‐opioid receptor antagonist and a kappa‐opioid receptor partial agonist to be administered when the risk of alcohol consumption is present. Nalmefene is not used in the clinic to any considerable extent and is—to the best of our knowledge—not recommended in clinical guidelines [9]. Other medications have been used off‐label to treat AUD, for example, ondansetron, topiramate, prazosin, gabapentin, varenicline and baclofen [2]. If assisted withdrawal treatment is needed, benzodiazepines should be provided, either as an inpatient or outpatient treatment, depending on the severity of symptoms and previous medical history [8]. It is estimated that 45%–90% of patients receiving AUD treatment relapse within the first 3 years of treatment [12, 13]. Relapse risk factors are age, health, the severity of AUD, abstinence duration, comorbid substance use disorder (SUD), smoking, unpleasant life events, stress, living alone, having no ‘life purpose’ and psychological factors, for example, insight, readiness to seek help, drinking goals and motivation [14]. The sparse treatment options with divergent results have led to a search for novel treatment strategies against AUD, and one of the molecular targets has been the glucagon‐like peptide‐1 (GLP‐1) receptor (GLP‐1R) [15].
2. The Addicted Brain
In 1954, Olds and Milner reported that rats voluntarily and repeatedly self‐stimulate specific brain areas electrically, that is, positive reinforcement [16]. In 1972, it was proposed that self‐stimulation activates dopamine‐containing neurons [17], and in 1993, the ‘incentive‐sensitisation theory of addiction’ was presented [18]. The theory differentiates between ‘wanting’ a drug, triggered by reward cues in addicted individuals and ‘liking’ a drug. The ‘wanting’ is believed to be generated in the dopaminergic mesolimbic system projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), and the ‘liking’ is generated in more discrete hedonic hotspots in the brain, not dependent on dopamine [19]. The consequence of chronic and heavy intake of alcohol and other drugs of abuse changes the brain reward system, and with continued use, impairment of function in brain areas associated with executive functions, motivated behaviour, stress control and emotionality, for example, the midbrain, prefrontal cortex and amygdala [20].
3. The Dopamine System
Dopamine is a catecholamine neurotransmitter synthesised from the amino acid tyrosine [21]. It is released into the synaptic cleft upon stimulation, binding to presynaptic and postsynaptic dopamine receptors. If dopamine doesn't bind to a receptor, it is broken down or transported back into the presynaptic neuron by the dopamine transporter (DAT) and then repacked into vesicles by the vesicular monoamine transporter 2 (VMAT2) for recycling or degradation (Figure 1) [22]. The plasma membrane protein DAT plays a pivotal role in brain dopamine homeostasis and is a target for many addictive drugs and therapeutics [23]. The central dopaminergic system contains dopaminergic neurons localised in the VTA and the substantia nigra, projecting to the NAc, amygdala, hippocampus, prefrontal cortex and dorsal striatum [24]. Disinhibition or stimulation of dopaminergic VTA neurons plays a critical role in the reinforcing effects of alcohol and other drugs of abuse [24]. Radioligand imaging studies in patients with AUD [25] have reported decreased dopamine receptor availability, indicating reduced brain dopamine function [26]. Whether this is caused by a primary dopaminergic mechanism of action or indirectly by changes in other neurotransmitter systems, for example, glutamate or GABA, has not been ruled out [27]. Post‐mortem brain studies [28, 29] and a single‐photon emission tomography (SPECT) study [30] have reported a significant reduction in striatal DAT availability in patients with AUD compared to healthy controls.
FIGURE 1.
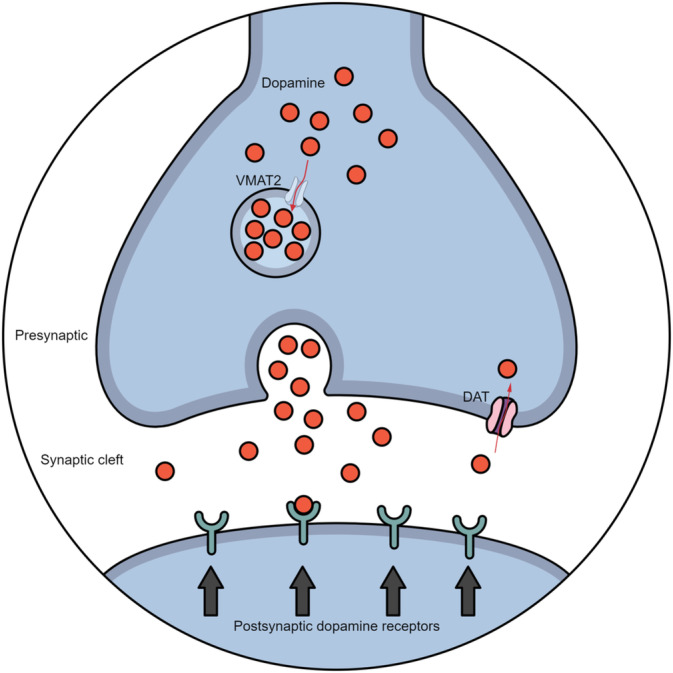
The dopamine synapse. Note: The dopamine synapse with its presynaptic and postsynaptic terminal of a dopamine neuron. Dopamine is synthesised in the presynaptic terminal. After the release into the synaptic cleft, it binds to postsynaptic or presynaptic receptors. The free synaptic dopamine, which does not bind to the dopamine receptor, is then broken down or recycled into the presynaptic neuron by the dopamine transporter (DAT), where it is repacked into vesicles by the vesicular monoamine transporter 2 (VMAT2) or catabolised.
4. GLP‐1
GLP‐1 is an endogenous 30‐amino acid peptide hormone produced by cleavage of the prohormone proglucagon. GLP‐1 is produced in the L‐cells in the small intestines and released in response to food intake. It is hastily inactivated (with a half‐life of 3–5 min) by the enzyme dipeptidyl peptidase‐4 (DPP‐4) [31]. GLP‐1 potentiates insulin secretion and suppresses glucagon secretion, regulating overall glycaemic control. It slows down gastric emptying and regulates appetite and food intake via appetite‐ and reward‐related areas of the brain [32, 33]. Importantly, rodent data show that GLP‐1 is also produced in the nucleus tractus solitarius (NTS) of the brain stem and released as a neurotransmitter in the VTA and NAc [34]. This is in concordance with preclinical data in rodents and non‐human primates showing expression of GLP‐1Rs in brain regions involved in reward and addiction, for example, VTA, NAc, septal nucleus, hypothalamus and amygdala [35, 36, 37, 38, 39, 40]. Human GLP‐1R mRNA positive cells are localised in the hypothalamus, hippocampus, thalamus, caudate–putamen, globus pallidum and cerebral cortex [41], and human post‐mortem brain studies have revealed the presence of GLP‐1R in the brainstem, hypothalamus, thalamus, amygdala, hippocampus and cerebral cortex [41, 42, 43]. In addition, GLP‐1 mRNA is significantly elevated in the hippocampus in individuals with AUD compared to healthy controls [43]. GLP‐1 protein expression is reported in most cortical areas and in the diencephalon and the brainstem [42].
5. GLP‐1 Receptor Agonists (GLP‐1RAs)
In 2006, the FDA approved the first GLP‐1RA, exenatide twice daily, to treat Type 2 diabetes. In 2011, exenatide once weekly was approved [44]. Since then, several GLP‐1RAs have been approved for the treatment of Type 2 diabetes, and in 2014, the first GLP‐1RA was approved for the treatment of obesity (body mass index [BMI] ≥ 30 kg/m2 or a BMI ≥ 27 kg/m2 and at least one weight‐related comorbid condition) [45]. In 2019, the first oral GLP‐1RA was approved to treat patients with Type 2 diabetes [46]. In line with the conception of a centrally mediated effect on appetite regulation, several preclinical studies report on the blood–brain barrier penetrance of GLP‐1 and GLP‐1RAs [47, 48, 49, 50], for example, the GLP‐1RA liraglutide was following fluorescently labelling detected in the arcuate nucleus and other hypothalamic areas in mice [50].
6. GLP‐1 and GLP‐1RAs—Preclinical Studies
6.1. Alcohol
Several GLP‐1RAs have been evaluated in preclinical addiction models regarding their effects on alcohol consumption in rodents and non‐human primates. In a conditioned place preference (CPP) model, preclinical trials report decreased or abolished alcohol place preference when animals are pretreated with systemically administered exenatide [51, 52], or exenatide is injected centrally into the NTS [53] or NAc [54]. The same results are reported for the GLP‐1RA liraglutide [55]. In a rodent two‐bottle‐choice paradigm, pretreatment with exenatide administered systemically [51, 52, 56], or injected centrally into the VTA [52, 57], NTS [53], NAc, dorsal hippocampus, lateral hypothalamus [57], NAc shell [54, 57] or laterodorsal tegmental area [54], is reported to reduce alcohol intake. It has also been reported that exenatide decreased alcohol consumption in a rodent operant self‐administration paradigm when administered systemically [51] or injected centrally into the VTA [58]. The effects of GLP‐1RAs on alcohol intake have also been tested in non‐human primates with long‐term access to alcohol, where alcohol consumption was significantly reduced when treated with the GLP‐1RAs exenatide or liraglutide compared to placebo [59]. Recently, the newer and more potent GLP‐1RA semaglutide was reported to reduce alcohol consumption in rats [60, 61] and non‐human primates as well [62].
6.2. Dopamine Homeostasis
GLP‐1‐producing neurons projecting from the NTS to the VTA and the core and shell regions of the NAc have been identified [34], and GLP‐1R stimulation in the NTS increases expression of dopamine‐related genes, e.g. mRNA encoding tyrosine hydroxylase, which is required for the synthesis of dopamine [21]. However, exenatide does not seem to alter the expression of dopamine receptors or DAT in the NAc [63]. Several preclinical studies have investigated how GLP‐1 modulates dopamine signalling. Microdialysis and fast‐scan cyclic voltammetry studies indicate attenuated alcohol‐induced dopamine release in the NAc following systemic injections of liraglutide [55], or exenatide [51], and after local injection of exenatide into NTS [53]. Exenatide also attenuates cocaine‐, amphetamine‐ and nicotine‐induced increases in NAc or lateral septal dopamine levels in rats [64, 65, 66, 67, 68]. However, GLP‐1RAs do not seem to suppress baseline dopamine levels, as opposed to their lowering effects on elevated dopamine levels induced by drugs of abuse, including alcohol [64, 67]. The mechanism by which GLP‐1R stimulation affects dopamine homeostasis is less clear. In rat brain slices from the lateral septum and striatum, GLP‐1R stimulation increases DAT expression [65, 69]. In the lateral septum, GLP‐1R stimulation reduces septal expression of the retrograde messenger 2‐arachidonylglycerol (2‐AG), as well as its metabolite, arachidonic acid [65]. Interestingly, arachidonic acid reduces septal DAT function, suggesting that arachidonic acid may be a novel regulator of central DA homeostasis [65]. However, other preclinical studies report unaffected striatal DAT availability after GLP‐1R stimulation in wild‐type and knock‐out mice [69], as well as in rat NAc [64].
7. GLP‐1 and GLP‐1RAs ‐ Human Studies
7.1. Alcohol
Clinical trials investigating the effects of alcohol on gastrointestinal (GI) hormones in healthy controls report no changes in plasma GLP‐1 levels after consumption of alcohol [70, 71, 72, 73] or after intravenous alcohol [70]. However, one study in patients diagnosed with Type 2 diabetes and consuming alcohol and a fat‐rich meal reported decreased postprandial GLP‐1 levels [74]. It is thus uncertain whether the result can be attributed to the consumption of alcohol or other nutrients. It has been reported that there is an increased prevalence of AUD among patients having bariatric surgery performed [75, 76], and postsurgery changes of several gut peptides, including GLP‐1, have been described [77, 78]. In a newly published review, it has been reported that postprandial GLP‐1 plasma levels increase after surgery and are associated with a more extensive weight loss, but fasting GLP‐1 levels do remain low [77]. However, due to the very short half‐life of peripherally released endogenous GLP‐1, it is unlikely, that it could reach GLP‐1Rs in reward‐related brain areas. Therefore, the effect of GLP‐1RAs on appetite regulation, and possible effect on alcohol consumption, is most likely due to a direct effect of GLP‐1RAs on GLP‐1R in reward‐related brain areas and not via peripherally released endogenous GLP‐1. The first report on GLP‐1RA‐related reduction in alcohol intake in humans was a conference abstract from 2011 [79]. The author performed a cross‐sectional study (only published in abstract format) conducted on individuals diagnosed with Type 2 diabetes and treated with liraglutide for 3 months, showing a reduction in alcohol consumption [79]. Recently, human data from several register studies have been published, and data is in concordance with the results from Kalra [79]. Both a Danish and an American nationwide register‐based studies have reported a lower risk of an alcohol‐related event or AUD diagnosis when individuals diagnosed with diabetes or obesity were treated with a GLP‐1RA [80, 81]. A case series of six individuals receiving semaglutide for obesity have reported a reduction in AUD symptomatology based on the Alcohol Use Disorders Identification Test (AUDIT) score [82]. Also, secondary analysis from an RCT investigating the GLP‐1RA dulaglutide as a treatment for nicotine dependence found that the dulaglutide group had a 29% reduction in alcohol consumption after 12 weeks of treatment with dulaglutide, compared to the placebo group, and this was not correlated with smoking status. The results showed no significant change in alcohol consumption in the group of participants being heavy drinkers. No data on calories or fluids consumed were reported [83]. Lastly, a social media study on posts related to GLP‐1 or GLP‐1/GIP receptor agonists has reported reductions in craving and desire to drink [84]. Recently, we published on the effects of the GLP‐1RA exenatide in patients receiving psychotherapy for AUD in a randomised, placebo‐controlled clinical trial [85]. No significant difference in the reduction of heavy drinking days was found. However, a subgroup of participants had a brain fMRI scan performed at baseline and Week 26. In a predefined region of interest (ROI) analysis, alcohol cue‐induced activation was significantly reduced in the ventral striatum, dorsal striatum and putamen in the exenatide group compared to the placebo group [85]. In the fMRI whole‐brain analyses, a significant reduction in alcohol cue‐induced activation in the left caudate and septal area was observed in the exenatide group compared to the placebo group [85]. In an exploratory analysis of BMI subgroups, a reduction in heavy drinking days and total alcohol intake was found in individuals with a baseline BMI ≥ 30 kg/m2 treated with exenatide, compared to the BMI‐matched placebo group (total n = 30). In the exenatide‐treated patients with a BMI ≥ 25 kg/m2, a significant reduction in total alcohol intake compared to the matched BMI placebo group was also found (total n = 75). In contrast, in patients with a normal BMI (18.5–24.9 kg/m2), the placebo group had a significantly larger reduction in heavy drinking days than the exenatide‐treated group (total n = 52) [85]. Table 1 gives an overview of all ongoing clinical trials investigating the effects of a GLP‐1RA in AUD. Table 2 gives an overview of all the clinical studies mentioned above, investigating the effects of a GLP‐1RA in AUD.
TABLE 1.
All unpublished or ongoing clinical trials investigating a GLP‐1 receptor agonist in alcohol use disorder registered until December 12, 2024.
| NCT Identifier | Drug | Administration | n = | Primary outcome | Expected end date |
|---|---|---|---|---|---|
| NCT05895643 | Semaglutide | sc, 2.4 mg once weekly | 108 | Change in heavy drinking days | December 2025 |
| NCT05520775 | Semaglutide | sc, 1.0 mg once weekly | 48 | Change in alcohol consumption in four laboratory sessions | Completed |
| NCT05891587 | Semaglutide | sc, 1.0 mg once weekly | 80 | Change in alcohol drinking measured per week | July 2025 |
| NCT05892432 | Semaglutide | po, 7.0 mg once daily | 135 | Change in alcohol craving | June 2025 |
| NCT06015893 | Semaglutide | sc, 2.4 mg once weekly | 52 | Change in alcohol consumption | December 2030 |
Abbreviation: NCT, ClinicalTrials.gov.
TABLE 2.
The most important studies referenced addressing GLP‐1 RA's and AUD.
| Authors | Human/animal | Design | Primary diagnose | Number of subjects | Type of GLP‐1RA | Main findings |
|---|---|---|---|---|---|---|
| Egecioglu et al., 2013 [51] | Male rodents | Intermittent access 20% alcohol two‐bottle‐choice drinking paradigm/operant alcohol self‐administration procedure | — | — | Exenatide | Attenuated alcohol‐induced locomotor stimulation and accumbal dopamine release. Conditioned place preference for alcohol was abolished by acute and chronic treatment with Ex4. Treatment decreased alcohol intake in an intermittent alcohol two‐bottle‐choice model and alcohol‐seeking behaviour |
| Shirazi et al., 2013 [52] | Male rodents | Intermittent access two‐bottle free‐choice drinking model/alcohol CPP test/microinjection into the VTA | — | — | Exenatide/antagonist exendin‐3 (9–39)/GLP‐1 (7–36) | GLP‐1 and exenatide reduced alcohol intake in an intermittent access two‐bottle free choice drinking model. Blockade of GLP‐1 receptors resulted in increased alcohol intake. GLP‐1 injection reduced alcohol reward in an alcohol CPP test. Direct stimulation of the VTA GLP‐1 receptors potently reduced alcohol intake |
| Vallöf et al., 2019 [53] | Male rodents | NTS‐GLP‐1R activation on alcohol‐induced locomotor stimulation, accumbal dopamine release and alcohol consumption/memory of alcohol reward in a CPP model | — | — | Exenatide | Exenatide in the NTS inhibits the acute effects of alcohol, as measured by alcohol‐induced locomotor stimulation, accumbal dopamine release and memory consolidation of alcohol reward in a CPP model. NTS‐exenatide dose‐dependently decreases alcohol intake |
| Vallöf et al., 2019 [54] | Rodents | Infusion of GLP‐1RA into NAcS, anterior VTA, posterior VTA or LDTg evaluated with an alcohol‐induced locomotor stimulation and memory of alcohol reward paradigm in a CPP model in mice, as well as on alcohol intake in high‐consuming rats | — | — | Exenatide | Ex4 into the NAcS blocks alcohol‐induced locomotor stimulation and memory of alcohol reward and decreases alcohol intake. The GLP‐1R expression in NAc is elevated in high compared to low alcohol‐consuming rats. Intra‐LDTg‐Ex4 attenuates alcohol‐induced locomotor stimulation and reduces alcohol intake but does not affect memory of alcohol reward |
| Vallöf et al., 2016 [55] | Rodents | Microdialysis in the NAc to measure dopamine levels after systemic administration of liraglutide/CPP/alcohol two‐bottle‐choice paradigm/operant alcohol self‐administration | — | — | Liraglutide | Acute treatment suppressed alcohol‐induced accumbal dopamine release and CPP in mice. Acute administration prevented the alcohol deprivation effect and reduced alcohol intake in rats, while repeated treatment decreased alcohol intake in rats and reduced operant self‐administration of alcohol |
| Sirohi et al., 2016 [56] | Mice | Alcohol intake following peripheral administration of Ex4 in FLOX and GLP‐1R KDNestin mice | — | — | Exenatide | Pretreatment selectively blocked alcohol consumption in the FLOX mice but was ineffective in altering alcohol intake in GLP‐1R KDNestin mice. |
| Colvin et al., 2020 [57] | Male rats | Directly injections in multiple sites throughout the prosencephalic and mesencephalic regions of the brain, evaluated by a two‐bottle choice intermittent access model | — | — | Exenatide | Results indicated that GLP‐1 receptor signalling effectively suppressed voluntary alcohol intake when injected into the VTA, NAcC and NAcS, the DMHipp and LH |
| Dixon et al., 2020 [58] | Male rats | Intermittent access to 20% alcohol and trained to nose poke for 20% alcohol/injections were given in VTA, and effects on self‐administration, motivation and relapse were assessed | — | — | Exenatide | Intra‐VTA injections significantly reduced alcohol self‐administration but did not reduce the reacquisition of alcohol self‐administration after extinction nor the motivation to obtain alcohol |
| Chuong et al., 2023 [60] | Male and female rodents | A drinking‐in‐the‐dark procedure to test the effects of semaglutide on binge‐like drinking behaviour/acute effects of semaglutide on sIPSCs from CeA and ILC neurons | — | — | Semaglutide | Semaglutide dose‐dependently reduced binge‐like alcohol drinking and dependence‐induced alcohol drinking. Semaglutide increased sIPSC frequency in CeA and ILC neurons from alcohol‐naïve rats, suggesting enhanced GABA release, but had no overall effect on GABA transmission in alcohol‐dependent rats |
| Aranäs et al., 2023 [61] | Male and female rodents | Intermittent access model (decrease alcohol intake and block relapse‐like drinking)/imaging the binding of fluorescently marked semaglutide to NAc/alcohol‐induced locomotor stimulation and in vivo dopamine release in NAc/in vivo release of dopamine metabolites (DOPAC and HVA) and gene expression of enzymes metabolising dopamine (MAOA and COMT) | — | — | Semaglutide | Acute and repeated semaglutide administration reduced alcohol intake and prevented relapse‐like drinking/semaglutide attenuated the ability of alcohol to cause hyperlocomotion and to elevate dopamine in NAc/semaglutide enhanced DOPAC and HVA in NAc when alcohol was onboard and increased the gene expression of COMT and MAOA |
| Thomsen et al., 2019 [59] | Male non‐human primates | Vehicle‐controlled study investigating voluntary alcohol drinking | — | 24 | Liraglutide/exenatide | Liraglutide and, to a lesser extent, exenatide significantly reduced alcohol consumption |
| Fink‐Jensen et al., 2024 [62] | Male non‐human primates | Vehicle‐controlled study investigating voluntary alcohol drinking | — | 20 | Semaglutide | Semaglutide significantly reduced alcohol consumption |
| Kalra, 2011 [79] | Humans | Cross‐sectional review | Type 2 diabetes | 42 | Liraglutide | Alcohol intake decreased markedly in 33 out of 42 patients, and nine subjects completely stopped alcohol consumption |
| Wium‐Andersen et al., 2022 [80] | Humans | A nationwide register‐based cohort and self‐controlled case series study (2009–2017) | Type 2 diabetes | 38.454 (GLP‐1RA)/49.222 (DPP‐4) | ATC‐code A10BJ | Initiation of GLP‐1 treatment was associated with a lower risk of an alcohol‐related event compared with initiation of DPP‐4 during the first 3 months of follow‐up |
| Wang et al., 2024 [81] | Humans | Retrospective cohort study of electronic health records | Type 2 diabetes/obesity | 83.825 (obesity)/598.803 (Type 2 diabetes) | Semaglutide | Semaglutide, compared with other antiobesity/diabetes medications, is associated with a 50%–56% lower risk for both the incidence and recurrence of AUD for a 12‐month follow‐up period |
| Richards et al., 2023 [82] | Humans | Case series—Retrospective chart review | Obesity | 6 | Semaglutide | Significant reduction in AUD symptomatology based on AUDIT score improvement |
| Probst et al., 2023 [83] | Humans | Secondary analysis of alcohol data from an RCT evaluating therapy for smoking cessation | Nicotine dependence | 255 | Dulaglutide | At Week 12, participants receiving dulaglutide drank 29% less than participants receiving a placebo |
| Quddos et al., 2023 [84] | Humans | Analysis of social media texts (Reddit)/remote study including alcohol drinkers with a BMI ≥ 30 self‐reported taking semaglutide, tirzepatide or no medication | Type 2 diabetes/obesity | 68.250 posts/153 participants | Semaglutide/tirzepatide | Among the alcohol‐related posts (n = 1580), 71% were identified as craving reduction, decreased desire to drink/significantly lower self‐reported intake of alcohol, drinks per drinking episode, binge drinking odds and AUDIT scores |
| Klausen et al., 2022 [85] | Humans | Randomised, placebo‐controlled clinical trial | Alcohol use disorder | 127 | Exenatide | Attenuated fMRI alcohol cue reactivity in the ventral striatum and septal area. Reduced DAT availability. Exploratory analyses showed significantly reduced heavy drinking days and total alcohol intake in a subgroup of obese patients |
Abbreviations: ATC, Anatomical Therapeutic Chemical; AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; CeA, central amygdala; COMT, catechol‐O‐methyltransferase; CPP, conditioned place preference; DAT, dopamine transporter; DMHipp, dorsomedial hippocampus; DOPAC, 3,4‐dihydroxyphenylacetic acid; DPP‐4, dipeptidyl peptidase‐4 inhibitor; Ex4, exenatide; FLOX, genetic ablation inserting loxP sites surrounding glp1r genes; GLP‐1R KDNestin, GLP‐1R selectively ablated from the central nervous system; HVA, homovanillic acid; ILC, infralimbic cortex; LDTg, laterodorsal tegmental area; LH, lateral hypothalamus; MAOA, monoamine oxidase‐A; NAc, nucleus accumbens; NAcC, nucleus accumbens core; NAcS, nucleus accumbens shell; NTS, nucleus tractus solitarius; RCT, randomised controlled trial; sIPSCs, spontaneous inhibitory postsynaptic currents; VTA, ventral tegmental area.
7.2. Dopamine Homeostasis
Three clinical trials have investigated the effects of GLP‐1RAs on DAT availability in humans; a randomised placebo‐controlled clinical trial in Parkinson's disease patients treated with exenatide once weekly reported no changes in DAT availability measured with a SPECT‐DAT‐scan after 48 weeks of treatment [86]. A smaller clinical SPECT study performed by our research group investigated DAT availability in 10 healthy volunteers with no record of AUD or SUDs. All participants received placebo and exenatide infusions while placed in the SPECT scanner for 100 min (40 min with saline infusion followed by 60 min of exenatide infusion) [69]. No acute changes in DAT availability were observed [69]. In the published exenatide AUD trial [85], a subgroup of the AUD patients had a SPECT‐DAT scan performed at baseline and at Week 26. At the Week 26 rescan, a significant reduction in DAT availability in the striatum, caudate and putamen was found in the exenatide group, compared to the placebo group [85]. When baseline values for the AUD patients were compared to a sample of healthy controls, no significant difference in baseline DAT availability between AUD patients and healthy controls was found [85].
8. Adverse Events and Safety
GLP‐1RAs are widely used, with an exposure of over 20 million patient‐years, equivalent to 20 million patients medicated with a GLP‐1RA for 1 year [87]. A common mild to moderate, transient side effect of most GLP‐1RAs is GI‐related. The GI side effects reported in the GLP‐1 exenatide AUD trial [85] were more pronounced than those previously reported in diabetes and obesity trials [88, 89], suggesting that AUD patients are more prone to GI adverse events [90]. Depressive disorders [91] and suicidal ideation [92] are often co‐occurring with AUD. During the last year, the scientific and regulatory society and the media have focused on a potentially increased suicide/suicidal ideation rate among patients receiving a GLP‐1RA [93]. Analyses from the FDA Adverse Event Reporting System (FAERS) have concluded that there is no causal link between GLP‐1RAs and suicidality [94], and a very recent retrospective cohort study of electronic health records among individuals without a diagnosis of AUD does not support a higher risk of suicidal ideation when treated with the GLP‐1RA semaglutide compared to other antidiabetes or obesity medications [95]. Patients with AUD have an increased risk for hepatic damage [1], and a rodent study has recently shown that the GLP‐1RA exenatide improves alcohol‐associated hepatic steatosis [96]. In individuals with nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), it is reported that the GLP‐1RA semaglutide causes a reduction in liver enzyme levels, reduces liver stiffness and improves metabolic parameters [97]. To the best of our knowledge, the role of GLP‐1RAs in alcohol‐related liver disease (ALD) has not yet been reported, but one clinical trial investigating this has been initiated [98]. The renal elimination of GLP‐1RAs [32] is also advantageous in AUD patients, and results from a post hoc analysis have indicated that semaglutide does reduce albuminuria and the risk of new‐onset macroalbuminuria [99]. Semaglutide has also been shown to reduce the incidence of death due to cardiovascular disease, nonfatal stroke and nonfatal myocardial infarction in nondiabetic patients with pre‐existing cardiovascular disease and a BMI of 27 or higher [100]. Heavy alcohol consumption is associated with reduced bone mass density and increased risk of bone fractures [101]. Several rodent studies have shown that GLP‐1RAs increase bone mass and enhance bone strength [102]. In humans, treatment with a GLP‐1RA does not seem to impact bone health [103]. In the exenatide AUD trial, no significant difference in bone marker levels between the two groups was observed, indicating that treatment with the GLP‐1RA exenatide did not increase the risk of bone fractures in this specific group of patients—at least not in a 6‐month treatment period [85]. Earlier studies [104] and case reports [105] have reported that GLP‐1RAs were associated with an increased risk of pancreatitis or pancreatic cancer in patients with Type 2 diabetes. This increased risk may have limited the enthusiasm for testing GLP‐1RAs against AUD, as patients with AUD are already at higher risk for pancreatitis and pancreatic cancer [92]. However, a systematic review and meta‐analysis including three high‐quality, randomised clinical trials and 18 700 patients diagnosed with diabetes and treated with GLP‐1RAs or placebo found no significant association [106]. These findings are supported by a recent meta‐analysis including more than 55,000 patients [107], as well as a study comparing the risk of pancreatitis [108] or pancreatic cancer [109] in patients treated with GLP‐1RAs and patients treated with other antidiabetic therapies. In the exenatide AUD trial [85], no elevated pancreatic plasma enzyme levels above the upper limit were observed, nor were any incidences of pancreatitis recorded [85]. In the two clinical nicotine trials, there were no registered incidences of pancreatitis [110, 111] nor in the cocaine trial [112]. Because only one published clinical trial in AUD patients has investigated the effects of GLP‐1RAs, recent safety concerns regarding treatment with GLP‐1RAs in AUD patients related to alcoholic ketoacidosis or hypoglycaemia are still to be explored. In individuals receiving therapy with a GLP‐1RA for the treatment of diabetes or obesity, the risk of hypoglycaemic episodes or ketoacidosis is dependent on comedication with a sulfonylurea [113]. Also, severe complications such as malnutrition, cirrhosis, and sarcopenia are still to be investigated, even though—to the best of our knowledge—no data support these concerns [114].
9. Discussion
Promising treatment effects of GLP‐1RAs on alcohol intake have been reported in preclinical trials [115], case stories [82], in register‐based cohort studies [80, 81], secondary analyses of data [83] and social media comments [84]. Still, the exenatide AUD trial—the only published RCT including patients with primarily AUD—showed that exenatide was not superior to placebo on the primary alcohol outcomes, except in a post hoc analysis of the subgroup of patients with comorbid obesity (BMI ≥ 30 kg/m2). However, in the subgroup of patients with BMI ≤ 25 kg/m2, the exenatide subgroup significantly increased the number of heavy drinking days compared to the placebo group. A possible explanation of the increased heavy drinking days in the lean exenatide subgroup could be that they experienced a more considerable exenatide‐induced decrease in blood sugar [116], leading to more alcohol cravings [117], causing more heavy drinking days [85]. In preclinical studies, high alcohol‐consuming animals are reported to decrease their alcohol intake more than low alcohol‐consuming animals when treated with GLP‐1RAs [52, 55]. The lack of effect of exenatide on the primary endpoints could be related to the severity profile of the study participants whose baseline alcohol intake was lower than what is reported in other clinical pharmacotherapy alcohol trials [118, 119].
Recently, papers have described that individuals treated with a GLP‐1RA for obesity have reduced their alcohol intake or alcohol‐related behaviour [80, 82, 83, 84, 120]. A plausible explanation could be that overlapping brain circuits are involved in obesity and in addiction [121]. The antiobesity effects of GLP‐1RAs may be due to a change in food preference [122], satiety signal [32] or that individuals with obesity have deranged GLP‐1 signalling [123], which might be due to changes in gene expression [124]. An fMRI study in obese individuals also reported a normalised brain response to food cues when treated with the GLP‐1RA exenatide [125].
The NAc, which is part of the ventral striatum, plays a pivotal role in addiction and relapse [126, 127, 128]. Repeated use of drugs can cause a permanently hypersensitive state to drug‐associated stimuli—‘incentive salience’ [21], causing addictive behaviour [18]. In the exenatide AUD trial, we found significantly reduced fMRI alcohol cue reactivity in the ventral striatum (and other brain areas), implying that exenatide‐treated patients with AUD experience less incentive salience of alcohol‐associated cues [85]. This has also been reported previously in an RCT investigating the effects of cue‐exposure training in patients with AUD [129].
In 1954, Olds and Milner reported that rodents with electrodes implanted in the septal area were conveying the highest reward response [16], and it has been reported that the lateral septum receives dopaminergic input [130]. A rodent study has also shown that GLP‐1Rs are highly expressed in the septum and that GLP‐1R stimulation might regulate addiction‐related effects by reducing dopamine levels via increased DAT expression [65]. The GLP‐1RA exenatide is reported to attenuate alcohol‐, cocaine‐, amphetamine‐ and nicotine‐induced increases in accumbal or lateral septal dopamine levels in rats [51, 64, 65, 66, 67, 68]. In accordance with these preclinical data, whole‐brain fMRI results from the exenatide AUD trial showed a significant reduction in alcohol cue reactivity in the septal area [85], indicating that the septal area may play a role in the effects of GLP‐1RAs on addictive behaviour.
In humans, data on dopamine and DAT availability, measured with different brain‐imaging modalities or in post‐mortem brains, are divergent [25, 26, 28, 30, 131, 132], and so are the results of GLP‐1RA‐induced DAT availability [69]. In individuals without AUD, no acute changes in DAT levels are reported following infusion of the GLP‐1RA exenatide [69]. The same was reported in Parkinson's disease patients treated with exenatide for 48 weeks [86]. In the human exenatide AUD trial, no significant baseline difference in DAT availability was observed between AUD patients and individuals without AUD [85]. After 26 weeks of treatment with exenatide, reduced DAT availability was observed in the striatum, caudate and putamen [85]. This GLP‐1RA‐induced reduction in DAT availability may counteract the decreased dopamine activity previously reported in patients with AUD [127]. Nevertheless, the divergent findings on DAT availability in preclinical and human studies add to the assumption that GLP‐1 regulation of DAT might be species‐dependent and that the precise mechanisms in different species are still to be elucidated [69, 115]. The effects of GLP‐1RAs might also be caused by changes in other neurotransmitter systems, for example, GABA [27], as indicated by a preclinical study, where treatment with the GLP‐1RA semaglutide enhanced GABA release in the central nucleus of amygdala and infralimbic cortex neurons in alcohol‐naïve rats. In alcohol‐dependent rats, a more heterogeneous response was observed with increased network‐dependent GABA release in some neurons and decreased GABA release in the remaining cells [60]. In conclusion, the central mechanisms of action involved in the potential effects of GLP‐1RAs in AUD are not fully elucidated. Newer and more potent GLP‐1RAs are now available for clinical use, and several randomised clinical trials involving different treatment durations and different GLP‐1RA doses have been initiated, which may pave the road for further investigation of the potential role of GLP‐1RAs in the medical treatment of AUD and other addictive disorders. However, the somewhat high prize of GLP‐1RAs poses barriers to treatment access, which may be an even greater challenge for patients with AUD compared to patients with diabetes and/or obesity.
Conflicts of Interest
M.K.K. has no conflict of interest. G.M.K. has received personal honoraria from H. Lundbeck, Sage Biogen and Sanos and serves as chair for SIAB in HBP (personal honorarium). T.V. has been part of speaker's bureaus, served on scientific advisory panels, served as a consultant to and/or received research support from Amgen, Boehringer Ingelheim, Eli Lilly, Gilead, AstraZeneca, Mundipharma, MSD/Merck, Novo Nordisk and SunPharmaceuticals. A.F.‐J. has received an unrestricted research grant from Novo Nordisk to investigate the effects of GLP‐1R stimulation on metabolic disturbances in patients with schizophrenia treated with antipsychotics and serves on an advisory panel for Novo Nordisk (no honorarium).
Acknowledgements
The Research Fund of the Mental Health Services, Capital Region of Denmark, and the Novo Nordisk Foundation funded the writing of this manuscript.
Funding: The Research Fund of the Mental Health Services, Capital Region of Denmark and the Novo Nordisk Foundation funded the writing of this manuscript.
Contributor Information
Mette Kruse Klausen, Email: mette.kruse.klausen@regionh.dk.
Anders Fink‐Jensen, Email: anders.fink-jensen@regionh.dk.
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analysed in this focused review.
References
- 1. Carvalho A. F., Heilig M., Perez A., Probst C., and Rehm J., “Alcohol Use Disorders,” Lancet 394 (2019): 10200, 10.1016/S0140-6736(19)31775-1. [DOI] [PubMed] [Google Scholar]
- 2. Witkiewitz K., Litten R. Z., and Leggio L., “Advances in the Science and Treatment of Alcohol Use Disorder,” Science Advances 5 (2019): eaax4043, 10.1126/sciadv.aax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO , “Global Status Report on Alcohol and Health 2018,” (2018).
- 4. Kohn R., Saxena S., Levav I., and Saraceno B., “The Treatment Gap in Mental Health Care,” Bulletin of the World Health Organization 82, no. 11 (2004): 858–866. [PMC free article] [PubMed] [Google Scholar]
- 5. Askgaard G., Leon D. A., Deleuran T., and Tolstrup J. S., “Hospital Admissions and Mortality in the 15 Years After a First‐Time Hospital Contact With an Alcohol Problem: A Prospective Cohort Study Using the Entire Danish Population,” International Journal of Epidemiology 49, no. 1 (2020): 94–102, 10.1093/ije/dyz159. [DOI] [PubMed] [Google Scholar]
- 6. Nutt D. J., King L. A., and Phillips L. D., “Drug Harms in the UK: A Multicriteria Decision Analysis,” Lancet 376 (2010): 1558–1565, 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- 7. Carroll K. M. and Kiluk B. D., “Cognitive Behavioral Interventions for Alcohol and Drug Use Disorders: Through the Stage Model and Back Again,” Psychology of Addictive Behaviors 31, no. 8 (2017): 847–861, 10.1037/adb0000311.Cognitive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence, Alcohol‐Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking (High‐Risk Drinking) and Alcohol Dependence: Clinical Guideline, Revised 2020, (2011), www.nice.org.uk/guidance/cg115. [PubMed] [Google Scholar]
- 9. Kranzler H. R. and Soyka M., “Diagnosis and Pharmacotherapy of Alcohol Use Disorder,” Journal of the American Medical Association 320, no. 8 (2018): 815–824, 10.1001/jama.2018.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plosker G. L., “Acamprosate: A Review of Its Use in Alcohol Dependence,” Drugs 75, no. 11 (2015): 1255–1268, 10.1007/s40265-015-0423-9. [DOI] [PubMed] [Google Scholar]
- 11. Shen W. W., “Anticraving Therapy for Alcohol Use Disorder: A Clinical Review,” Neuropsychopharmacology Reports 38, no. 3 (2018): 105–116, 10.1002/npr2.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bottlender M. and Soyka M., “Outpatient Alcoholism Treatment: Predictors of Outcome After 3 Years,” Drug and Alcohol Dependence 80, no. 1 (2005): 83–89, 10.1016/j.drugalcdep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 13. Connor J. P., Haber P. S., and Hall W. D., “Alcohol Use Disorders,” Lancet 387, no. 10022 (2016): 988–998, 10.1016/S0140-6736(15)00122-1. [DOI] [PubMed] [Google Scholar]
- 14. Sliedrecht W., de Waart R., Witkiewitz K., and Roozen H. G., “Alcohol Use Disorder Relapse Factors: A Systematic Review,” Psychiatry Research 278 (2019): 97–115, 10.1016/j.psychres.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 15. Skibicka K. P., “The Central GLP‐1: Implications for Food and Drug Reward,” Frontiers in Neuroscience 7 (2013): 1–10, 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olds J. and Milner P., “Positive Reinforcement Produced by Electrical Stimulation of Septal Area and Other Regions of rat Brain,” Journal of Comparative and Physiological Psychology 47, no. 6 (1954): 419–427, 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 17. Crow T. J., “A Map of the Rat Mesencephalon for Electrical Self‐Stimulation,” Brain Research 36, no. 2 (1972): 265–273, 10.1016/0006-8993(72)90734-2. [DOI] [PubMed] [Google Scholar]
- 18. Robinson T. E. and Berridge K. C., “The Neural Basis of Drug Craving: An Incentive‐Sensitization Theory of Addiction,” Brain Research Reviews 18, no. 3 (1993): 247–291, 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- 19. Berridge K. C. and Robinson T. E., “Liking, Wanting, and the Incentive‐Sensitization Theory of Addiction,” American Psychologist 71, no. 8 (2016): 670–679, 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volkow N. D. and Morales M., “The Brain on Drugs: From Reward to Addiction,” Cell 162, no. 4 (2015): 712–725, 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 21. Daubner S. C., Le T., and Wang S., “Tyrosine Hydroxylase and Regulation of Dopamine Synthesis,” Archives of Biochemistry and Biophysics 508, no. 1 (2011): 1–12, 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McHugh P. C. and Buckley D. A., “The Structure and Function of the Dopamine Transporter and Its Role in CNS Diseases,” in Vitamins and Hormones, vol. 98, 1st ed. (Elsevier Inc, 2015): 339–369, 10.1016/bs.vh.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 23. Vaughan R. A. and Foster J. D., “Mechanisms of Dopamine Transporter Regulation in Normal and Disease States,” Trends in Pharmacological Sciences 34, no. 9 (2013): 489–496, 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Volkow N. D., Wise R. A., and Baler R., “The Dopamine Motive System: Implications for Drug and Food Addiction,” Nature Reviews. Neuroscience 18, no. 12 (2017): 741–752, 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- 25. Volkow N. D., Wang G. J., Fowler J. S., et al., “Decreases in Dopamine Receptors but Not in Dopamine Transporters in Alcoholics,” Alcoholism, Clinical and Experimental Research 20, no. 9 (1996): 1594–1598, 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 26. Nutt D. J., Lingford‐Hughes A., Erritzoe D., and Stokes P. R. A., “The Dopamine Theory of Addiction: 40 Years of Highs and Lows,” Nature Reviews. Neuroscience 16, no. 5 (2015): 305–312, 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 27. Volkow N. D., Wang G. J., Fowler J. S., Tomasi D., and Telang F., “Addiction: Beyond Dopamine Reward Circuitry,” Proceedings of the National Academy of Sciences of the United States of America 108, no. 37 (2011): 15037–15042, 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirth N., Meinhardt M. W., Noori H. R., et al., “Convergent Evidence From Alcohol‐Dependent Humans and Rats for a Hyperdopaminergic State in Protracted Abstinence,” Proceedings of the National Academy of Sciences 113, no. 11 (2016): 3024–3029, 10.1073/pnas.1506012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tupala E., Kuikka J. T., Hall H., et al., “Measurement of the Striatal Dopamine Transporter Density and Heterogeneity in Type 1 Alcoholics Using Human Whole Hemisphere Autoradiography,” NeuroImage 14 (2001): 87–94, 10.1006/nimg.2001.0793. [DOI] [PubMed] [Google Scholar]
- 30. Yen C. H., Yeh Y. W., Liang C. S., et al., “Reduced Dopamine Transporter Availability and Neurocognitive Deficits in Male Patients With Alcohol Dependence,” PLoS ONE 10, no. 6 (2015): 1–14, 10.1371/journal.pone.0131017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vilsbøll T., Agersø H., Krarup T., and Holst J. J., “Similar Elimination Rates of Glucagon‐Like Peptide‐1 in Obese Type 2 Diabetic Patients and Healthy Subjects,” Journal of Clinical Endocrinology and Metabolism 88, no. 1 (2003): 220–224, 10.1210/jc.2002-021053. [DOI] [PubMed] [Google Scholar]
- 32. Holst J. J., “The Physiology of Glucagon‐Like Peptide 1,” Physiological Reviews 87, no. 4 (2007): 1409–1439, 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 33. Müller T. D., Finan B., Bloom S. R., et al., “Glucagon‐Like Peptide 1 (GLP‐1),” Molecular Metabolism 30 (2019): 72–130, 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alhadeff A. L., Rupprecht L. E., and Hayes M. R., “GLP‐1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake,” Endocrinology 153, no. 2 (2012): 647–658, 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cork S. C., Richards J. E., Holt M. K., Gribble F. M., Reimann F., and Trapp S., “Distribution and Characterisation of Glucagon‐Like Peptide‐1 Receptor Expressing Cells in the Mouse Brain,” Molecular Metabolism 4, no. 10 (2015): 718–731, 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heppner K. M., Kirigiti M., Secher A., et al., “Expression and Distribution of Glucagon‐Like Peptide‐1 Receptor mRNA, Protein and Binding in the Male Nonhuman Primate (Macaca mulatta) Brain,” Endocrinology 156, no. 1 (2015): 255–267, 10.1210/en.2014-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jensen C. B., Pyke C., Rasch M. G., Dahl A. B., Knudsen L. B., and Secher A., “Characterization of the Glucagonlike Peptide‐1 Receptor in Male Mouse Brain Using a Novel Antibody and In Situ Hybridization,” Endocrinology 159, no. 2 (2018): 665–675, 10.1210/en.2017-00812. [DOI] [PubMed] [Google Scholar]
- 38. Merchenthaler I., Lane M., and Shughrue P., “Distribution of Pre‐Pro‐Glucagon and Glucagon‐Like Peptide‐1 Receptor Messenger RNAs in the Rat Central Nervous System,” Journal of Comparative Neurology 403, no. 2 (1999): 261–280. [DOI] [PubMed] [Google Scholar]
- 39. Rinaman L., “Ascending Projections From the Caudal Visceral Nucleus of the Solitary Tract to Brain Regions Involved in Food Intake and Energy Expenditure,” Brain Research 1350, no. 412 (2010): 18–34, 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vrang N. and Grove K., “The Brainstem Preproglucagon System in a Non‐Human Primate (Macaca mulatta),” Brain Research 1397 (2011): 28–37, 10.1016/j.brainres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 41. Alvarez E., Martínez M. D., Roncero I., et al., “The Expression of GLP‐1 Receptor mRNA and Protein Allows the Effect of GLP‐1 on Glucose Metabolism in the Human Hypothalamus and Brainstem,” Journal of Neurochemistry 92, no. 4 (2005): 798–806, 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 42. Gupta T., Kaur M., Shekhawat D., Aggarwal R., Nanda N., and Sahni D., “Investigating the Glucagon‐Like Peptide‐1 and Its Receptor in Human Brain: Distribution of Expression, Functional Implications, Age‐Related Changes and Species Specific Characteristics,” Basic and Clinical Neuroscience 14, no. 3 (2023): 341–354, 10.32598/bcn.2021.2554.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farokhnia M., Browning B. D., Crozier M. E., Sun H., Akhlaghi F., and Leggio L., “The Glucagon‐Like Peptide‐1 System Is Modulated by Acute and Chronic Alcohol Exposure: Findings From Human Laboratory Experiments and a Post‐Mortem Brain Study,” Addiction Biology 27, no. 5 (2022): 1–11, 10.1111/adb.13211. [DOI] [PubMed] [Google Scholar]
- 44. Knop F. K., Brønden A., and Vilsbøll T., “Exenatide: Pharmacokinetics, Clinical Use, and Future Directions,” Expert Opinion on Pharmacotherapy 18, no. 6 (2017): 555–571, 10.1080/14656566.2017.1282463. [DOI] [PubMed] [Google Scholar]
- 45. Nuffer W. A. and Trujillo J. M., “Liraglutide: A New Option for the Treatment of Obesity,” Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 35, no. 10 (2015): 926–934, 10.1002/phar.1639. [DOI] [PubMed] [Google Scholar]
- 46. Anderson S. L., Beutel T. R., and Trujillo J. M., “Oral Semaglutide in Type 2 Diabetes,” Journal of Diabetes and its Complications 34, no. 4 (2020): 107520, 10.1016/j.jdiacomp.2019.107520. [DOI] [PubMed] [Google Scholar]
- 47. Gabery S., Salinas C. G., Paulsen S. J., et al., “Semaglutide Lowers Body Weight in Rodents via Distributed Neural Pathways,” JCI Insight 5, no. 6 (2020): e133429, 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kastin A. J., Akerstrom V., and Pan W., “Interactions of Glucagon‐Like Peptide‐1 (GLP‐1) With the Blood‐Brain Barrier,” Journal of Molecular Neuroscience 18 (2002): 7–14. [DOI] [PubMed] [Google Scholar]
- 49. Salinas C. B. G., Lu T. T. H., Gabery S., et al., “Integrated Brain Atlas for Unbiased Mapping of Nervous System Effects Following Liraglutide Treatment,” Scientific Reports 8, no. 1 (2018): 1–12, 10.1038/s41598-018-28496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Secher A., Jelsing J., Baquero A. F., et al., “The Arcuate Nucleus Mediates GLP‐1 Receptor Agonist Liraglutide‐Dependent Weight Loss,” Journal of Clinical Investigation 124, no. 10 (2014): 4473–4488, 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egecioglu E., Steensland P., Fredriksson I., Feltmann K., Engel J. A., and Jerlhag E., “The Glucagon‐Like Peptide 1 Analogue Exendin‐4 Attenuates Alcohol Mediated Behaviors in Rodents,” Psychoneuroendocrinology 38, no. 8 (2013): 1259–1270, 10.1016/j.psyneuen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 52. Shirazi R. H., Dickson S. L., and Skibicka K. P., “Gut Peptide GLP‐1 and Its Analogue, Exendin‐4, Decrease Alcohol Intake and Reward,” PLoS ONE 8, no. 4 (2013): 1–7, 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vallöf D., Vestlund J., and Jerlhag E., “Glucagon‐Like Peptide‐1 Receptors Within the Nucleus of the Solitary Tract Regulate Alcohol‐Mediated Behaviors in Rodents,” Neuropharmacology 149 (2019): 124–132, 10.1016/j.neuropharm.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 54. Vallöf D., Kalafateli A. L., and Jerlhag E., “Brain Region Specific Glucagon‐Like Peptide‐1 Receptors Regulate Alcohol‐Induced Behaviors in Rodents,” Psychoneuroendocrinology 103 (2019): 284–295, 10.1016/j.psyneuen.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 55. Vallöf D., MacCioni P., Colombo G., et al., “The Glucagon‐Like Peptide 1 Receptor Agonist Liraglutide Attenuates the Reinforcing Properties of Alcohol in Rodents,” Addiction Biology 21, no. 2 (2016): 422–437, 10.1111/adb.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sirohi S., Schurdak J. D., Seeley R. J., Benoit S. C., and Davis J. F., “Central & Peripheral Glucagon‐Like Peptide‐1 Receptor Signaling Differentially Regulate Addictive Behaviors,” Physiology & Behavior 161 (2016): 140–144, 10.1016/j.physbeh.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 57. Colvin K. J., Killen H. S., Kanter M. J., Halperin M. C., Currie P. J., and Engel L., “Brain Site‐Specific Inhibitory Effects of the GLP‐1 Analogue Exendin‐4 on Alcohol Intake and Operant Responding for Palatable Food,” International Journal of Molecular Sciences 21, no. 24 (2020): 1–15, 10.3390/ijms21249710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dixon T. N., McNally G. P., and Ong Z. Y., “Glucagon‐Like Peptide‐1 Receptor Signaling in the Ventral Tegmental Area Reduces Alcohol Self‐Administration in Male Rats,” Alcoholism, Clinical and Experimental Research 44, no. 10 (2020): 2118–2129, 10.1111/acer.14437. [DOI] [PubMed] [Google Scholar]
- 59. Thomsen M., Holst J. J., Molander A., Linnet K., Ptito M., and Fink‐Jensen A., “Effects of Glucagon‐Like Peptide 1 Analogs on Alcohol Intake in Alcohol‐Preferring Vervet Monkeys,” Psychopharmacology 236, no. 2 (2019): 603–611, 10.1007/s00213-018-5089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chuong V., Farokhnia M., Khom S., et al., “The Glucagon‐Like Peptide‐1 (GLP‐1) Analogue Semaglutide Reduces Alcohol Drinking and Modulates Central GABA Neurotransmission,” JCI Insight 8, no. 12 (2023): e170671, 10.1172/jci.insight.170671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aranäs C., Edvardsson C. E., Shevchouk O. T., et al., “Semaglutide Reduces Alcohol Intake and Relapse‐Like Drinking in Male and Female Rats,” eBioMedicine 93 (2023): 104642, 10.1016/j.ebiom.2023.104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fink‐Jensen A., Wörtwein G., Klausen M. K., et al., “Effect of the Glucagon‐Like Peptide‐1 (GLP‐1) Receptor Agonist Semaglutide on Alcohol Consumption in Alcohol‐Preferring Male Vervet Monkeys,” Psychopharmacology 242 (2024): 63–70, 10.1007/s00213-024-06637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Richard J. E., Anderberg R. H., Göteson A., Gribble F. M., Reimann F., and Skibicka K. P., “Activation of the GLP‐1 Receptors in the Nucleus of the Solitary Tract Reduces Food Reward Behavior and Targets the Mesolimbic System,” PLoS ONE 10, no. 3 (2015): 1–21, 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fortin S. M. and Roitman M. F., “Central GLP‐1 Receptor Activation Modulates Cocaine‐Evoked Phasic Dopamine Signaling in the Nucleus Accumbens Core,” Physiology & Behavior 176, no. 3 (2017): 17–25, 10.1016/j.physbeh.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reddy I. A., Pino J. A., Weikop P., et al., “Glucagon‐Like Peptide 1 Receptor Activation Regulates Cocaine Actions and Dopamine Homeostasis in the Lateral Septum by Decreasing Arachidonic Acid Levels,” Translational Psychiatry 6, no. 5 (2016): e809, 10.1038/tp.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sørensen G., Reddy I. A., Weikop P., et al., “The Glucagon‐Like Peptide 1 (GLP‐1) Receptor Agonist Exendin‐4 Reduces Cocaine Self‐Administration in Mice,” Physiology & Behavior 149 (2015): 262–268, 10.1016/j.physbeh.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Egecioglu E., Engel J. A., and Jerlhag E., “The Glucagon‐Like Peptide 1 Analogue, Exendin‐4, Attenuates the Rewarding Properties of Psychostimulant Drugs in Mice,” PLoS ONE 8, no. 7 (2013): 1–7, 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Egecioglu E., Engel J. A., and Jerlhag E., “The Glucagon‐Like Peptide 1 Analogue Exendin‐4 Attenuates the Nicotine‐Induced Locomotor Stimulation, Accumbal Dopamine Release, Conditioned Place Preference as Well as the Expression of Locomotor Sensitization in Mice,” PLoS ONE 8, no. 10 (2013): 1–7, 10.1371/journal.pone.0077284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jensen M. E., Galli A., Thomsen M., et al., “Glucagon‐Like Peptide‐1 Receptor Regulation of Basal Dopamine Transporter Activity Is Species‐Dependent,” Neurochemistry International 138 (2020): 104772, 10.1016/j.neuint.2020.104772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lanng A. R., Gasbjerg L. S., Bergmann N. C., et al., “Gluco‐Metabolic Effects of Oral and Intravenous Alcohol Administration in Men,” Endocrine Connections 8, no. 10 (2019): 1372–1382, 10.1530/EC-19-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Svartberg J., Holst J. J., Gutniak M., and Adner N., “The Ethanol Augmentation of Glucose‐Induced Insulin Secretion Is Abolished by Calcium Antagonism With Nifedipine: No Evidence for a Role of Glucagon‐Like Peptide‐1 (GLP‐1),” Pancreas 16, no. 1 (1998): 66–71, 10.1097/00006676-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 72. Calissendorff J., Gustafsson T., Holst J. J., Brismar K., and Röjdmark S., “Alcohol Intake and Its Effect on Some Appetite‐Regulating Hormones in Man: Influence of Gastroprotection With Sucralfate,” Endocrine Research 37, no. 3 (2012): 154–162, 10.3109/07435800.2012.662662. [DOI] [PubMed] [Google Scholar]
- 73. Abraham K. A., Kearney M. L., Reynolds L. J., and Thyfault J. P., “Red Wine Enhances Glucose‐Dependent Insulinotropic Peptide (GIP) and Insulin Responses in Type 2 Diabetes During an Oral Glucose Tolerance Test,” Diabetology International 7, no. 2 (2016): 173–180, 10.1007/s13340-015-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dalgaard M., Thomsen C., Rasmussen B. M., Holst J. J., and Hermansen K., “Ethanol With a Mixed Meal Decreases the Incretin Levels Early Postprandially and Increases Postprandial Lipemia in Type 2 Diabetic Patients,” Metabolism 53, no. 1 (2004): 77–83, 10.1016/j.metabol.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 75. Bramming M., Becker U., Jørgensen M. B., Neermark S., Bisgaard T., and Tolstrup J. S., “Bariatric Surgery and Risk of Alcohol Use Disorder: A Register‐Based Cohort Study,” International Journal of Epidemiology 49, no. 6 (2021): 1826–1835, 10.1093/ije/dyaa147. [DOI] [PubMed] [Google Scholar]
- 76. Ostlund M. P., Backman O., Marsk R., et al., “Increased Admission for Alcohol Dependence After Gastric Bypass Surgery Compared With Restrictive Bariatric Surgery,” JAMA Surgery 148, no. 4 (2013): 374–377, 10.1001/jamasurg.2013.700. [DOI] [PubMed] [Google Scholar]
- 77. Çalık Başaran N., Dotan I., and Dicker D., “Post Metabolic Bariatric Surgery Weight Regain: The Importance of GLP‐1 Levels,” International Journal of Obesity (2024): 1–6, 10.1038/s41366-024-01461-2. [DOI] [PubMed] [Google Scholar]
- 78. Hutch C. R. and Sandoval D., “The Role of GLP‐1 in the Metabolic Success of Bariatric Surgery,” Endocrinology 158, no. 12 (2017): 4139–4151, 10.1210/en.2017-00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kalra S., “Change in Alcohol Consumption Following Liraglutide Initiation: A Real‐Life Experience,” in 71st American Diabetes Association Annual Meeting 2011, Poster 1029, vol. 93, (Alexandria, VA: American Diabetes Association, 2011). [Google Scholar]
- 80. Wium‐Andersen I. K., Wium‐Andersen M. K., Fink‐Jensen A., Rungby J., Jørgensen M. B., and Osler M., “Use of GLP‐1 Receptor Agonists and Subsequent Risk of Alcohol‐Related Events. A Nationwide Register‐Based Cohort and Self‐Controlled Case Series Study,” Basic & Clinical Pharmacology & Toxicology 131, no. 5 (2022): 372–379, 10.1111/bcpt.13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang W., Volkow N. D., Berger N. A., Davis P. B., Kaelber D. C., and Xu R., “Associations of Semaglutide With Incidence and Recurrence of Alcohol Use Disorder in Real‐World Population,” Nature Communications 15, no. 1 (2024): 4548, 10.1038/s41467-024-48780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Richards J. R., Dorand M. F., Royal K., Mnajjed L., Paszkowiak M., and Simmons W. K., “Significant Decrease in Alcohol Use Disorder Symptoms Secondary to Semaglutide Therapy for Weight Loss,” Journal of Clinical Psychiatry 85, no. 1 (2023): 1–5, 10.4088/JCP.23m15068. [DOI] [PubMed] [Google Scholar]
- 83. Probst L., Monnerat S., Vogt D. R., et al., “Effects of Dulaglutide on Alcohol Consumption During Smoking Cessation,” JCI Insight 8, no. 22 (2023): e170419, 10.1172/jci.insight.170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Quddos F., Hubshman Z., Tegge A., et al., “Semaglutide and Tirzepatide Reduce Alcohol Consumption in Individuals With Obesity,” Scientific Reports 13, no. 1 (2023): 1–12, 10.1038/s41598-023-48267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Klausen M. K., Jensen M. E., Møller M., et al., “Exenatide Once Weekly for Alcohol Use Disorder Investigated in a Randomized, Placebo‐Controlled Clinical Trial,” JCI Insight 7, no. 19 (2022): e159863, 10.1172/jci.insight.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Athauda D., Maclagan K., Skene S. S., et al., “Exenatide Once Weekly Versus Placebo in Parkinson's Disease: A Randomised, Double‐Blind, Placebo‐Controlled Trial,” Lancet 390, no. 10103 (2017): 1664–1675, 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. European Medicines Agency , “EMA Statement on Ongoing Review of GLP‐1 Receptor Agonists,” https://www.ema.europa.eu/en/news/ema‐statement‐ongoing‐review‐glp‐1‐receptor‐agonists.
- 88. Drucker D. J., Buse J. B., Taylor K., et al., “Exenatide Once Weekly Versus Twice Daily for the Treatment of Type 2 Diabetes: A Randomised, Open‐Label, Non‐Inferiority Study,” Lancet 372, no. 9645 (2008): 1240–1250, 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 89. MacConell L., Gurney K., Malloy J., Zhou M., and Kolterman O., “Safety and Tolerability of Exenatide Once Weekly in Patients With Type 2 Diabetes: An Integrated Analysis of 4,328 Patients,” Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 8 (2015): 241–253, 10.2147/DMSO.S77290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Haber P. S. and Kortt N. C., “Alcohol Use Disorder and the Gut,” Addiction 116, no. 3 (2021): 658–667, 10.1111/add.15147. [DOI] [PubMed] [Google Scholar]
- 91. Kathryn Mchugh R. and Weiss R. D., “Alcohol Use Disorder and Depressive Disorders,” Alcohol Research: Current Reviews 40, no. 1 (2019): e1–e8, 10.35946/arcr.v40.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rehm J., Gmel G. E., Gmel G., et al., “The Relationship Between Different Dimensions of Alcohol Use and the Burden of Disease—An Update,” Addiction 112, no. 6 (2017): 968–1001, 10.1111/add.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McIntyre R. S., “Glucagon‐Like Peptide‐1 Receptor Agonists (GLP‐1 RAs) and Suicidality: What Do We Know and Future Vistas,” Expert Opinion on Drug Safety 23, no. 5 (2024): 539–542, 10.1080/14740338.2024.2335215. [DOI] [PubMed] [Google Scholar]
- 94. McIntyre R. S., Mansur R. B., Rosenblat J. D., and Kwan A. T. H., “The Association Between Glucagon‐Like Peptide‐1 Receptor Agonists (GLP‐1 RAs) and Suicidality: Reports to the Food and Drug Administration Adverse Event Reporting System (FAERS),” Expert Opinion on Drug Safety 23, no. 1 (2024): 47–55, 10.1080/14740338.2023.2295397. [DOI] [PubMed] [Google Scholar]
- 95. Wang W., Volkow N. D., Berger N. A., Davis P. B., Kaelber D. C., and Xu R., “Association of Semaglutide With Risk of Suicidal Ideation in a Real‐World Cohort,” Nature Medicine 30, no. 1 (2024): 168–176, 10.1038/s41591-023-02672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mahalingam S., Bellamkonda R., Arumugam M. K., et al., “Glucagon‐Like Peptide 1 Receptor Agonist, Exendin‐4, Reduces Alcohol‐Associated Fatty Liver Disease,” Biochemical Pharmacology 213 (2023): 115613, 10.1016/j.bcp.2023.115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bandyopadhyay S., Das S., Samajdar S. S., and Joshi S. R., “Role of Semaglutide in the Treatment of Nonalcoholic Fatty Liver Disease or Non‐Alcoholic Steatohepatitis: A Systematic Review and Meta‐Analysis,” Diabetes and Metabolic Syndrome: Clinical Research & Reviews 17, no. 10 (2023): 102849, 10.1016/j.dsx.2023.102849. [DOI] [PubMed] [Google Scholar]
- 98.“Effects of NNC0194‐0499, Cagrilintide, and Semaglutide Alone or in Combinations on Liver Damage and Alcohol Use in People With Alcohol‐Related Liver Disease,” https://clinicaltrials.gov/study/NCT06409130.
- 99. Heerspink H. J. L., Apperloo E., Davies M., et al., “Effects of Semaglutide on Albuminuria and Kidney Function in People With Overweight or Obesity With or Without Type 2 Diabetes: Exploratory Analysis From the STEP 1, 2, and 3 Trials,” Diabetes Care 46, no. 4 (2023): 801–810, 10.2337/dc22-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lincoff A. M., Brown‐Frandsen K., Colhoun H. M., et al., “Semaglutide and Cardiovascular Outcomes in Obesity Without Diabetes,” New England Journal of Medicine 389 (2023): 2221–2232, 10.1056/nejmoa2307563. [DOI] [PubMed] [Google Scholar]
- 101. Gaddini G. W., Turner R. T., Grant K. A., and Iwaniec U. T., “Alcohol: A Simple Nutrient With Complex Actions on Bone in the Adult Skeleton,” Alcoholism, Clinical and Experimental Research 40, no. 4 (2016): 657–671, 10.1111/acer.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mabilleau G., Pereira M., and Chenu C., “Novel Skeletal Effects of Glucagon‐Like Peptide‐1 (GLP‐1) Receptor Agonists,” Journal of Endocrinology 236, no. 1 (2018): R29–R42, 10.1530/JOE-17-0278. [DOI] [PubMed] [Google Scholar]
- 103. Daniilopoulou I., Vlachou E., Lambrou G. I., et al., “The Impact of GLP1 Agonists on Bone Metabolism: A Systematic Review,” Medicina 58, no. 2 (2022): 1–13, 10.3390/medicina58020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Elashoff M., Matveyenko A. V., Gier B., Elashoff R., and Butler P. C., “Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon‐Like Peptide‐1–Based Therapies,” Gastroenterology 141, no. 1 (2011): 150–156, 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chis B. A. and Fodor D., “Acute Pancreatitis During GLP‐1 Receptor Agonist Treatment. A Case Report,” Clujul Medical 91, no. 1 (2017): 117–119, 10.15386/cjmed-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Storgaard H., Cold F., Gluud L. L., Vilsbøll T., and Knop F. K., “Glucagon‐Like Peptide‐1 Agonists and Risk of Acute Pancreatitis in Patients With Type 2 Diabetes,” Diabetes, Obesity and Metabolism 19, no. 6 (2017): 906–908, 10.1111/dom.12885. [DOI] [PubMed] [Google Scholar]
- 107. Abd El Aziz M., Cahyadi O., Meier J. J., Schmidt W. E., and Nauck M. A., “Incretin‐Based Glucose‐Lowering Medications and the Risk of Acute Pancreatitis and Malignancies: A Meta‐Analysis Based on Cardiovascular Outcomes Trials,” Diabetes, Obesity and Metabolism 22, no. 4 (2020): 699–704, 10.1111/dom.13924. [DOI] [PubMed] [Google Scholar]
- 108. Azoulay L., Filion K. B., Platt R. W., et al., “Association Between Incretin‐Based Drugs and the Risk of Acute Pancreatitis,” JAMA Internal Medicine 176, no. 10 (2016): 1464–1473, 10.1001/jamainternmed.2016.1522. [DOI] [PubMed] [Google Scholar]
- 109. Azoulay L., Filion K. B., Platt R. W., et al., “Incretin Based Drugs and the Risk of Pancreatic Cancer: International Multicentre Cohort Study,” BMJ 352 (2016): i581, 10.1136/bmj.i581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yammine L., Green C. E., Kosten T. R., et al., “Exenatide Adjunct to Nicotine Patch Facilitates Smoking Cessation and May Reduce Post‐Cessation Weight Gain: A Pilot Randomized Controlled Trial,” Nicotine & Tobacco Research 23, no. 10 (2021): 1682–1690, 10.1093/ntr/ntab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lengsfeld S., Burkard T., Meienberg A., et al., “Effect of Dulaglutide in Promoting Abstinence During Smoking Cessation: A Single‐Centre, Randomized, Double‐Blind, Placebo‐Controlled, Parallel Group Trial,” eClinicalMedicine 57 (2023): 101865, 10.1016/j.eclinm.2023.101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Angarita G. A., Matuskey D., Pittman B., et al., “Testing the Effects of the GLP‐1 Receptor Agonist Exenatide on Cocaine Self‐Administration and Subjective Responses in Humans With Cocaine Use Disorder,” Drug and Alcohol Dependence 221 (2021): 108614, 10.1016/j.drugalcdep.2021.108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nauck M. A., Quast D. R., Wefers J., and Meier J. J., “GLP‐1 Receptor Agonists in the Treatment of Type 2 Diabetes—State‐of‐the‐Art,” Molecular Metabolism 46 (2021): 101102, 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Conte C., Hall K. D., and Klein S., “Is Weight Loss–Induced Muscle Mass Loss Clinically Relevant?,” Journal of the American Medical Association 332, no. 1 (2024): 9–10, 10.1001/jama.2024.6586. [DOI] [PubMed] [Google Scholar]
- 115. Klausen M. K., Thomsen M., Wortwein G., and Fink‐Jensen A., “The Role of Glucagon‐Like Peptide 1 (GLP‐1) in Addictive Disorders,” British Journal of Pharmacology 179, no. 4 (2022): 625–641, 10.1111/bph.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Edwards C. M. B., Stanley S. A., Davis R., et al., “Exendin‐4 Reduces Fasting and Postprandial Glucose and Decreases Energy Intake in Healthy Volunteers,” American Journal of Physiology‐Endocrinology And Metabolism 281 (2001): E155–E161, 10.1152/ajpendo.2001.281.1.e155. [DOI] [PubMed] [Google Scholar]
- 117. Biery J. R., Williford J., and McMullen E. A., “Alcohol Craving in Rehabilitation: Assessment of Nutrition Therapy,” Journal of the American Dietetic Association 91, no. 4 (1991): 463–466. [PubMed] [Google Scholar]
- 118. Anton R. F., O'Malley S. S., Ciraulo D. A., et al., “Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence: The COMBINE Study: A Randomized Controlled Trial,” Journal of the American Medical Association 295, no. 17 (2006): 2003–2017, 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 119. Johnson B. A., Ait‐Daoud N., Bowden C. L., et al., “Oral Topiramate for Treatment of Alcohol Dependence: A Randomised Controlled Trial,” Lancet 361, no. 9370 (2003): 1677–1685, 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 120. Leggio L., Hendershot C. S., Farokhnia M., et al., “GLP‐1 Receptor Agonists Are Promising but Unproven Treatments for Alcohol and Substance Use Disorders,” Nature Medicine 29 (2023): 2993–2995, 10.1038/s41591-023-02634-8. [DOI] [PubMed] [Google Scholar]
- 121. Volkow N. D., Wang G. J., Tomasi D., and Baler R. D., “Obesity and Addiction: Neurobiological Overlaps,” Obesity Reviews 14, no. 1 (2013): 2–18, 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kadouh H., Chedid V., Halawi H., et al., “GLP‐1 Analog Modulates Appetite, Taste Preference, Gut Hormones, and Regional Body fat Stores in Adults With Obesity,” Journal of Clinical Endocrinology and Metabolism 105, no. 5 (2020): 1552–1563, 10.1210/clinem/dgz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Anandhakrishnan A. and Korbonits M., “Glucagon‐Like Peptide 1 in the Pathophysiology and Pharmacotherapy of Clinical Obesity,” World Journal of Diabetes 7, no. 20 (2016): 572–598, 10.4239/wjd.v7.i20.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mansur R. B., Fries G. R., Trevizol A. P., et al., “The Effect of Body Mass Index on Glucagon‐Like Peptide Receptor Gene Expression in the Post Mortem Brain From Individuals With Mood and Psychotic Disorders,” European Neuropsychopharmacology 29, no. 1 (2019): 137–146, 10.1016/j.euroneuro.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Van Bloemendaal L., IJzerman R. G., ten Kulve J. S., et al., “GLP‐1 Receptor Activation Modulates Appetite‐ and Reward‐Related Brain Areas in Humans,” Diabetes 63, no. 12 (2014): 4186–4196, 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 126. Martinez D., Gil R., Slifstein M., et al., “Alcohol Dependence Is Associated With Blunted Dopamine Transmission in the Ventral Striatum,” Biological Psychiatry 58, no. 10 (2005): 779–786, 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 127. Volkow N. D., Wang G. J., Telang F., et al., “Profound Decreases in Dopamine Release in Striatum in Detoxified Alcoholics: Possible Orbitofrontal Involvement,” Journal of Neuroscience 27, no. 46 (2007): 12700–12706, 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Everitt B. J. and Robbins T. W., “Drug Addiction: Updating Actions to Habits to Compulsions Ten Years on,” Annual Review of Psychology 67, no. 1 (2016): 23–50, 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- 129. Vollstädt‐Klein S., Loeber S., Kirsch M., et al., “Effects of Cue‐Exposure Treatment on Neural Cue Reactivity in Alcohol Dependence: A Randomized Trial,” Biological Psychiatry 69, no. 11 (2011): 1060–1066, 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 130. Sheehan T. P., Chambers R. A., and Russell D. S., “Regulation of Affect by the Lateral Septum: Implications for Neuropsychiatry,” Brain Research Reviews 46, no. 1 (2004): 71–117, 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 131. Volkow N. D., Chang L., Wang G. J., et al., “Low Level of Brain Dopamine D2 Receptors in Methamphetamine Abusers: Association With Metabolism in the Orbitofrontal Cortex,” American Journal of Psychiatry 158, no. 12 (2001): 2015–2021, 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 132. Volkow N. D., Fowler J. S., Wang G.‐J., et al., “Decreased Dopamine D2 Receptor Availability Is Associated With Reduced Frontal Metabolism in Cocaine Abusers,” Synapse 14, no. 2 (1993): 169–177, 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analysed in this focused review.


