Abstract
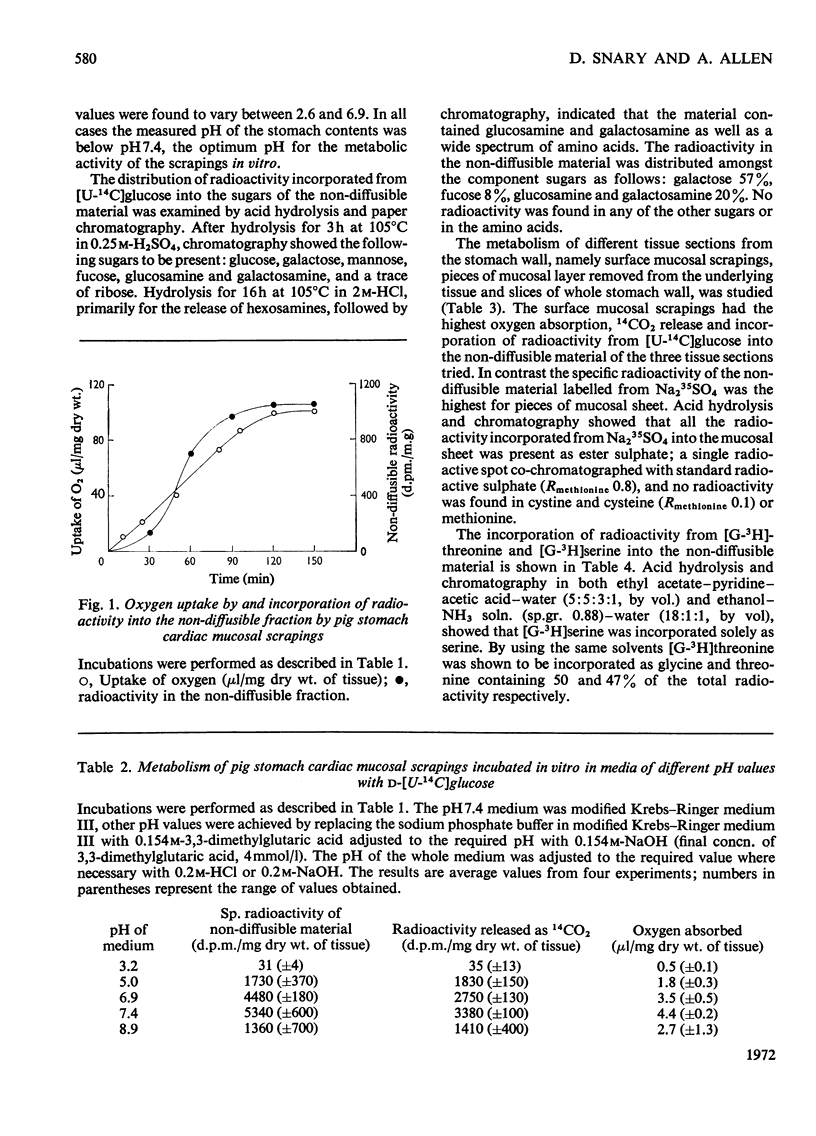
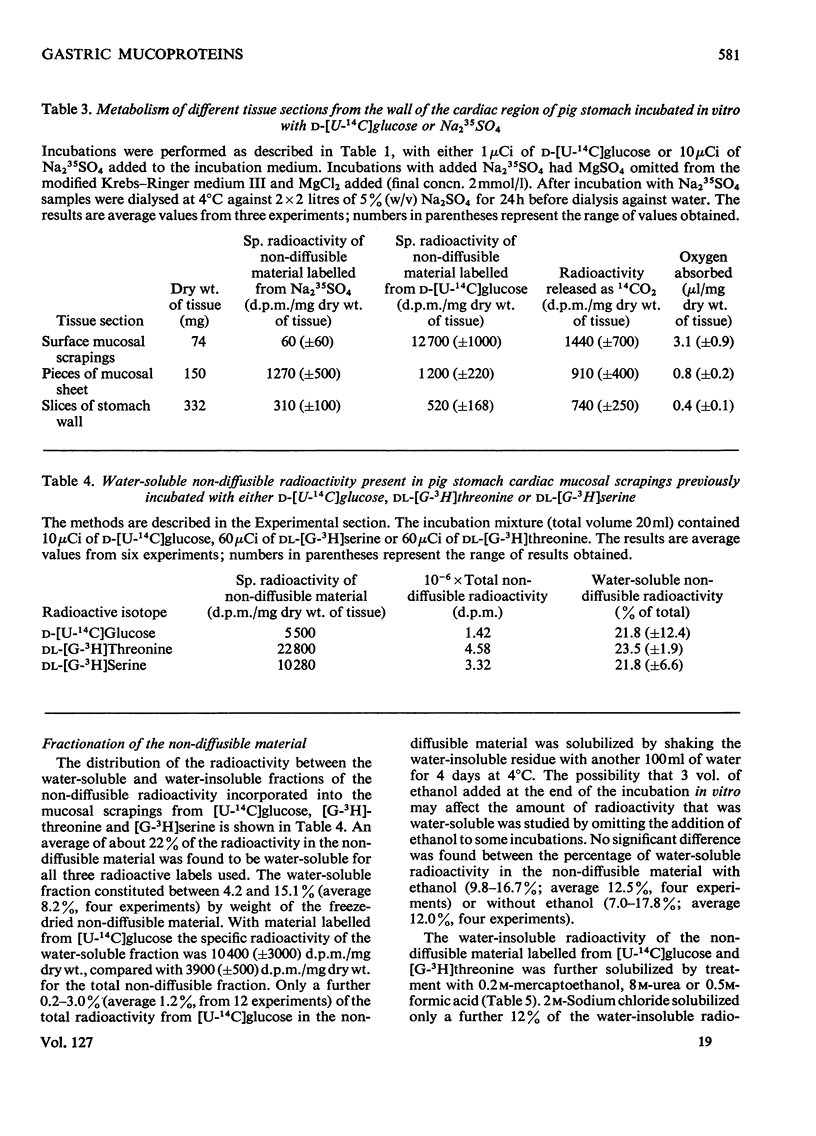
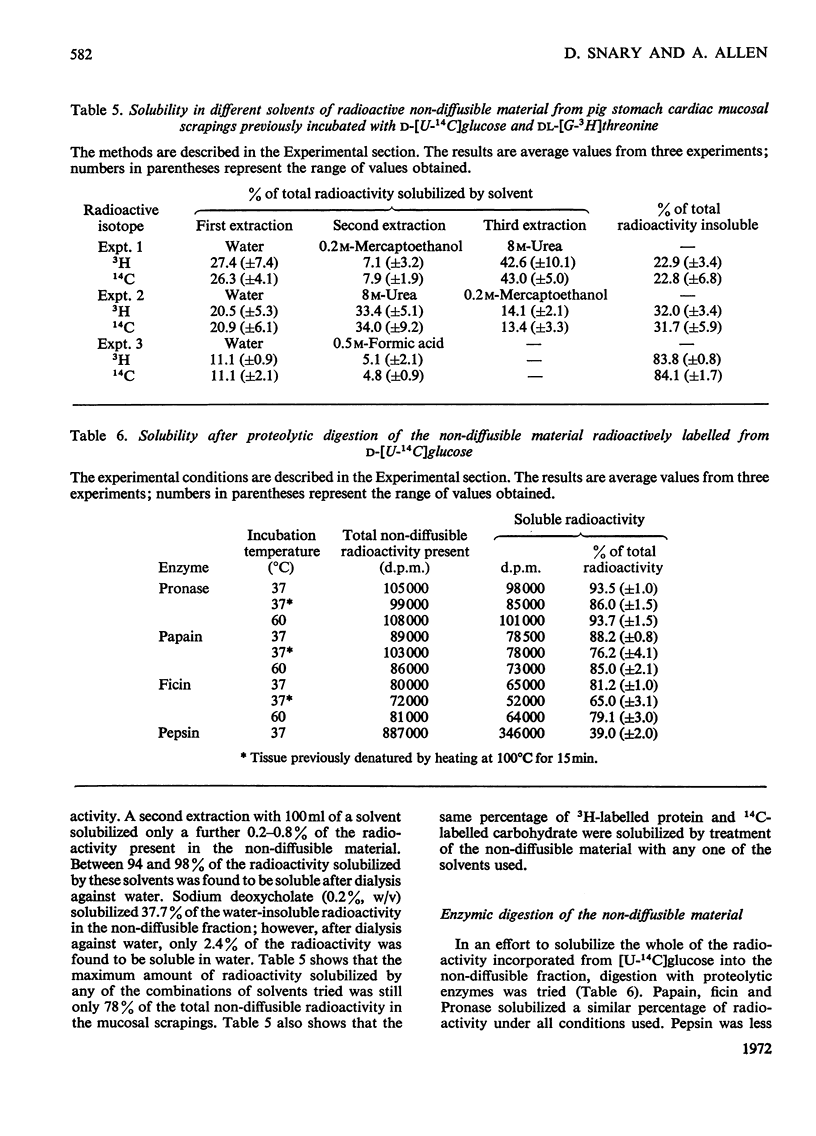
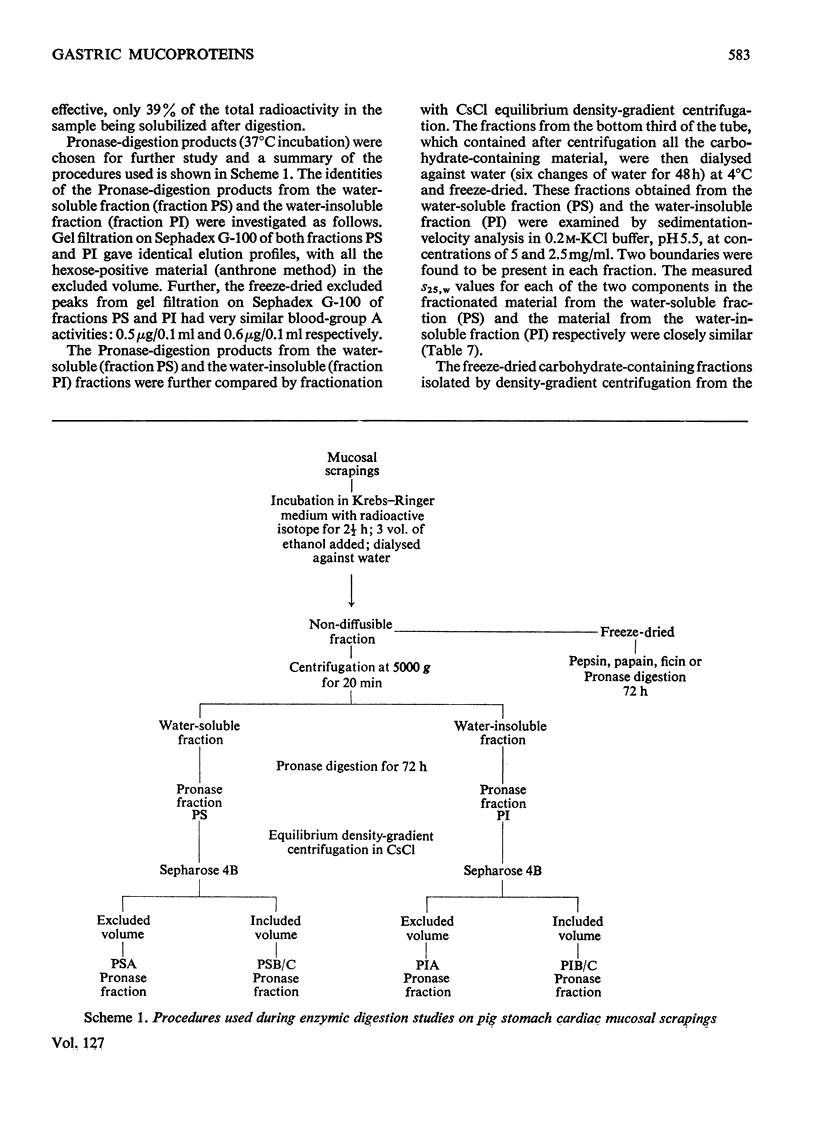
1. Optimum conditions, including the effect of media of different pH values, were determined for the incorporation of radioactive precursors into mucoproteins by pig gastric mucosa in vitro. 2. Mucosal scrapings incorporated radioactivity from [U-14C]-glucose and from [G-3H]threonine or [G-3H]serine solely into the carbohydrate and protein portions respectively of the mucoprotein molecules. 3. Of the radioactive mucoprotein 22% was water-soluble and up to 80% of the remainder was soluble in other solvents. 4. Pronase was the most successful proteolytic enzyme tested for making the mucoprotein water-soluble, up to 94% dissolving after digestion. 5. The Pronase digestion products of the mucoproteins were separated from protein by equilibrium-density-gradient centrifugation in a CsCl gradient. 6. These Pronase-digested mucoproteins were further fractionated on Sepharose 4B and the isolated fractions analysed by chemical and sedimentation-velocity methods. 7. Pronase digestion and solvent extraction of mucosal scrapings labelled with 14C in the carbohydrate and 3H in the protein showed that one type of mucoprotein was the only non-diffusible biosynthetic product of the scrapings in vitro, and that this mucoprotein was the only mucoprotein constituent of the water-soluble and water-insoluble mucus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A., Kent P. W. Biosynthesis of intestinal mucins. Effect of puromycin on mucoprotein biosynthesis by sheep colonic mucosal tissue. Biochem J. 1968 Jan;106(1):301–309. doi: 10.1042/bj1060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNET A., BESCOL-LIVERSAC J., GUILLAM C. LES MUCOPOLYSACCHARIDES DE LA MUQUEUSE GASTRIQUE HUMAINE. ETUDE HISTOCHIMIQUE ET HISTOAUTORADIOGRAPHIQUE, R'EALIS'EE AVEC LE 35-S-SULFATE SUR DES FRAGMENTS PR'ELEV'ES PAR BIOPSIE. I. LA MUQUEUSE GASTRIQUE 'A L''ETAT NORMAL. Arch Mal Appar Dig Mal Nutr. 1964 Apr;53:377–384. [PubMed] [Google Scholar]
- Creeth J. M., Denborough M. A. The use of equilibrium-density-gradient methods for the preparation and characterization of blood-group-specific glycoproteins. Biochem J. 1970 May;117(5):879–891. doi: 10.1042/bj1170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Kent P. W. Biosynthesis of intestinal mucins. 4. Utilization of [1-C]glucose by sheep colonic mucosa in vitro. Biochem J. 1963 Feb;86(2):248–254. doi: 10.1042/bj0860248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER F. G., NEBEL H. J. Nachweis und Bestimmung von Glucosamin und Galaktosamin auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1955 Sep 21;302(1):10–19. [PubMed] [Google Scholar]
- FLOREY H. W. The secretion and function of intestinal mucus. Gastroenterology. 1962 Sep;43:326–329. [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kent P. W., Allen A. The biosynthesis of intestinal mucins. The effect of salicylate on glycoprotein biosynthesis by sheep colonic and human gastric mucosal tissues in vitro. Biochem J. 1968 Feb;106(3):645–658. doi: 10.1042/bj1060645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Watanabe T., Nagai Y. Studies on acid mucopolysaccharides of pig gastric mucin. I. Fukushima J Med Sci. 1964 Dec;11(1):71–83. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludowieg J., Benmaman J. D. Colorimetric differentiation of hexosamines. Anal Biochem. 1967 Apr;19(1):80–88. doi: 10.1016/0003-2697(67)90136-4. [DOI] [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. Degradation of blood group-specific mucopoly-saccharides by ficin and papain. Nature. 1958 Sep 6;182(4636):648–650. doi: 10.1038/182648a0. [DOI] [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. Studies in immunochemistry. 20. The action of papain and ficin on blood-group-specific substances. Biochem J. 1961 Dec;81:639–647. doi: 10.1042/bj0810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer T., Glass G. B., Horowitz M. I. Purification and characterization of sulfated glycoproteins and hyaluronidase-resistant mucopolysaccharides from dog gastric mucosa. Biochemistry. 1968 Nov;7(11):3821–3829. doi: 10.1021/bi00851a006. [DOI] [PubMed] [Google Scholar]
- Race C., Ziderman D., Watkins W. M. An alpha-d-galactosyltransferase associated with the blood-group B character. Biochem J. 1968 May;107(5):733–735. doi: 10.1042/bj1070733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Schauer R., Schoop H. J., Faillard H. Zur Biosynthese der Glykolyl-Gruppe der N-Glykolyl-neuraminsaure. Die oxydative Umwandlung der N-Acetyl-Gruppe zur Glykolyl-Gruppe. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):645–652. [PubMed] [Google Scholar]
- Schrager J., Oates M. D. The isolation and partial characterisation of the principal gastric glycoprotein of 'visible' mucus. Digestion. 1971;4(1):1–12. doi: 10.1159/000197091. [DOI] [PubMed] [Google Scholar]
- Skoryna S. C., Waldron-Edward D. Physicochemical properties of gastric mucus and its role in the pathogenesis of gastric lesions. Ann N Y Acad Sci. 1967 Jan 26;140(2):835–847. doi: 10.1111/j.1749-6632.1967.tb51006.x. [DOI] [PubMed] [Google Scholar]
- Snary D., Allen A., Pain R. H. Structural studies on gastric mucoproteins: lowering of molecular weight after reduction with 2-mercaptoethanol. Biochem Biophys Res Commun. 1970 Aug 24;40(4):844–851. doi: 10.1016/0006-291x(70)90980-0. [DOI] [PubMed] [Google Scholar]
- Snary D., Allen A., Pain R. H. The structure of pig gastric mucus. Conformational transitions induced by salt. Eur J Biochem. 1971 Dec 22;24(1):183–189. doi: 10.1111/j.1432-1033.1971.tb19669.x. [DOI] [PubMed] [Google Scholar]
- Snary D., Allen A. Studies on gastric mucoproteins. The isolation and characterization of the mucoprotein of the water-soluble mucus from pig cardiac gastric mucosa. Biochem J. 1971 Aug;123(5):845–853. doi: 10.1042/bj1230845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIMER H. E., MOSHIN J. R. Serum glyco protein concentrations in experimental tuberculosis of guinea pigs. Am Rev Tuberc. 1953 Oct;68(4):594–602. doi: 10.1164/art.1953.68.4.594. [DOI] [PubMed] [Google Scholar]


