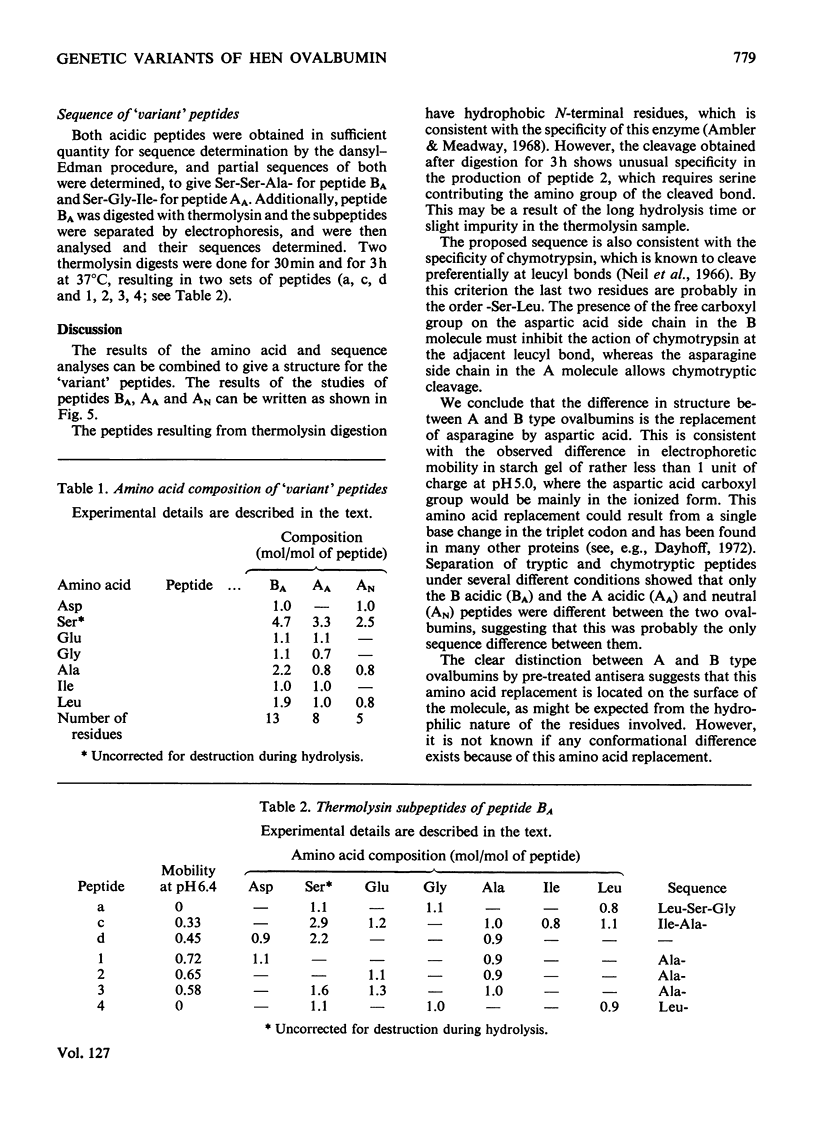
Abstract
Two forms of hen ovalbumin that exist as genetic variants were compared by physical and immunochemical techniques. The two ovalbumins could be distinguished by electrophoretic mobility and by antisera that had been pretreated with heterologous antigen. Peptide `maps' of chymotrypsin digests of the two ovalbumins revealed that three of the peptides were different. These were isolated and analysed. One form of ovalbumin (type B) contains the sequence: -Ser-Ser-Ala-Asp-Leu-Ser-Gly-Ile-Ala-Glu-Ser(Ser,Leu)- whereas the other form (type A) contains an asparagine residue in place of the aspartic acid residue. The -Asn-Leu-Ser- sequence of type A is not glycosylated. Chymotrypsin readily cleaved the leucine–serine bond in the asparagine-containing peptide, but not in the aspartic acid-containing variant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Meadway R. J. The use of thermolysin in amino acid sequence determination. Biochem J. 1968 Aug;108(5):893–895. doi: 10.1042/bj1080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., CLOUSE R. W., FORD J. D. HETEROGENEITY OF THE CARBOHYDRATE MOIETY OF CRYSTALLINE OVALBUMIN. Biochim Biophys Acta. 1963 Oct 29;78:379–381. doi: 10.1016/0006-3002(63)91652-4. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., NUENKE B. J., STRAYHORN W. D. Sulfhydryl content and tryptic susceptibility of thermally denatured ovalbumin. J Biol Chem. 1957 Oct;228(2):835–845. [PubMed] [Google Scholar]
- Fothergill L. A., Fothergill J. E. Immunochemical comparison of ovalbumins from nine different species. Comp Biochem Physiol A Comp Physiol. 1971 Oct;40(2):445–451. doi: 10.1016/0300-9629(71)90035-1. [DOI] [PubMed] [Google Scholar]
- Fothergill L. A., Fothergill J. E. Structural comparison of ovalbumins from nine different species. Eur J Biochem. 1970 Dec;17(3):529–532. doi: 10.1111/j.1432-1033.1970.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Fothergill L. A., Fothergill J. E. Thiol and disulphide contents of hen ovalbumin. C-terminal sequence and location of disulphide bond. Biochem J. 1970 Feb;116(4):555–561. doi: 10.1042/bj1160555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Hunt L. T., Dayhoff M. O. The occurrence in proteins of the tripeptides Asn-X-Ser and Asn-X-Thr and of bound carbohydrate. Biochem Biophys Res Commun. 1970 May 22;39(4):757–765. doi: 10.1016/0006-291x(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Hirs C. H. The primary structure of porcine pancreatic ribonuclease. I. The distribution and sites of carbohydrate attachment. J Biol Chem. 1970 Feb 10;245(3):624–636. [PubMed] [Google Scholar]
- KAMINSKI M. [Immunochemical study of the proteolysis of ovalbumin]. Ann Inst Pasteur (Paris) 1960 Jan;98:51–69. [PubMed] [Google Scholar]
- Neil G. L., Niemann C., Hein G. E. Structural specificity of alpha-chymotrypsin: polypeptide substrates. Nature. 1966 May 28;210(5039):903–907. doi: 10.1038/210903a0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PERLMANN G. E. Enzymatic dephosphorylation of ovalbumin and plakalbumin. J Gen Physiol. 1952 May;35(5):711–726. doi: 10.1085/jgp.35.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The isolation and properties of a fragment of bovine-serum albumin which retains the ability to combine with rabbit antiserum. Biochem J. 1957 Aug;66(4):677–686. doi: 10.1042/bj0660677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


