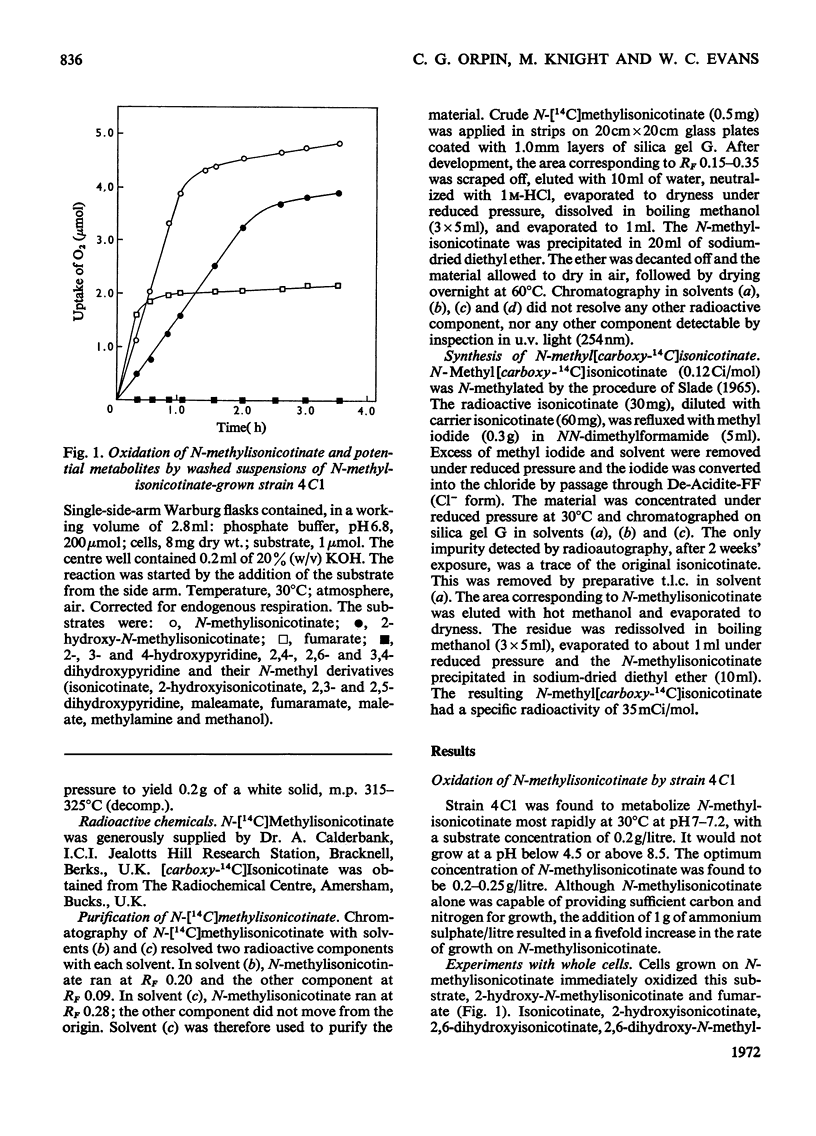
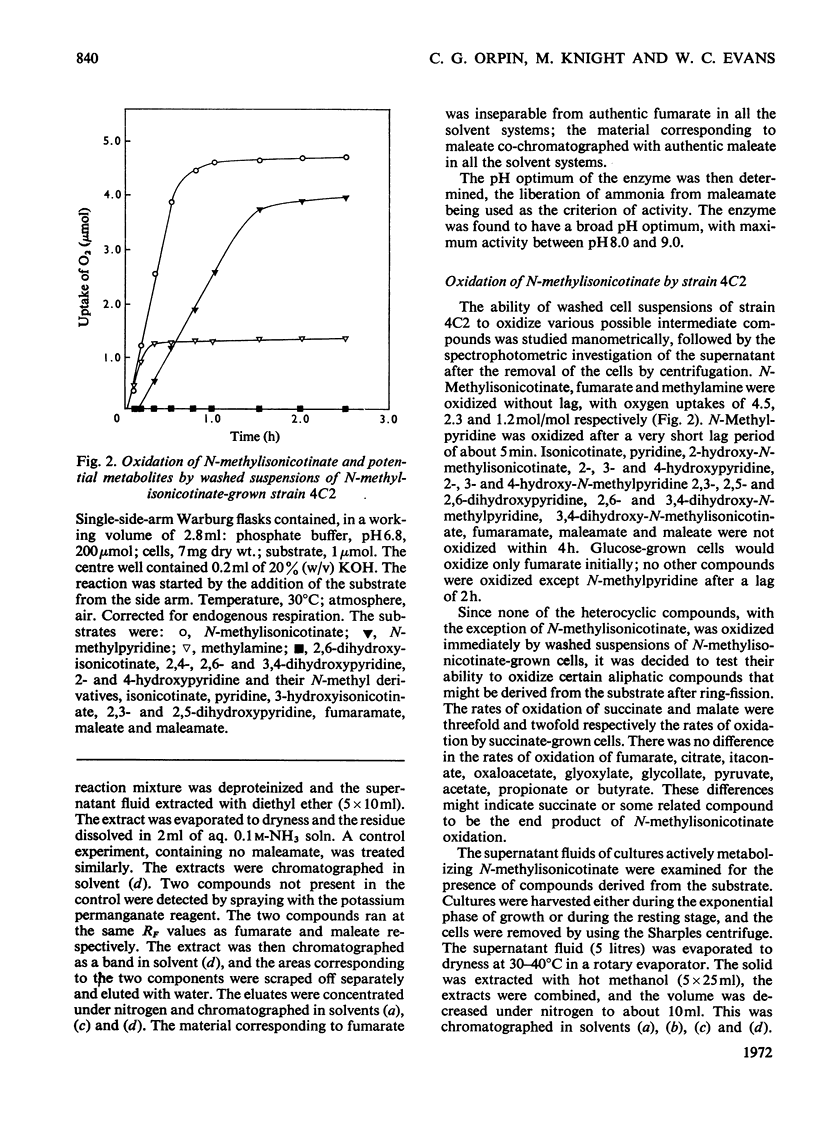
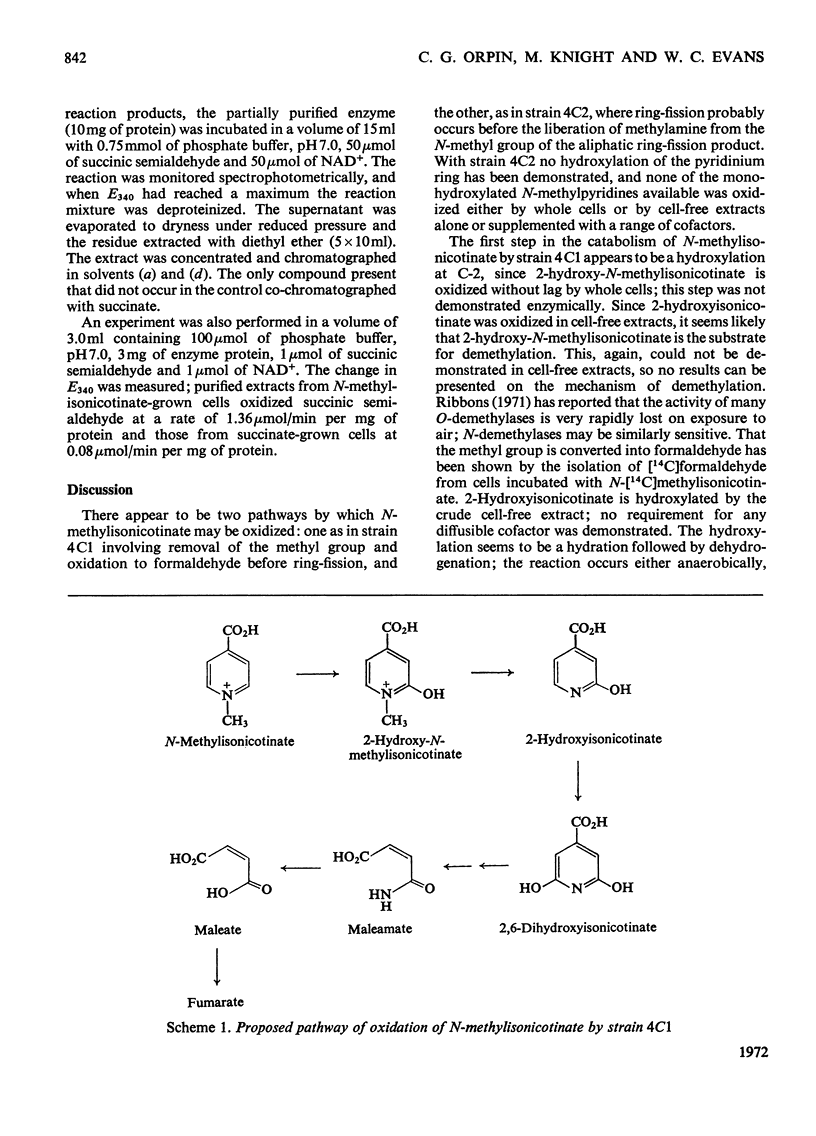
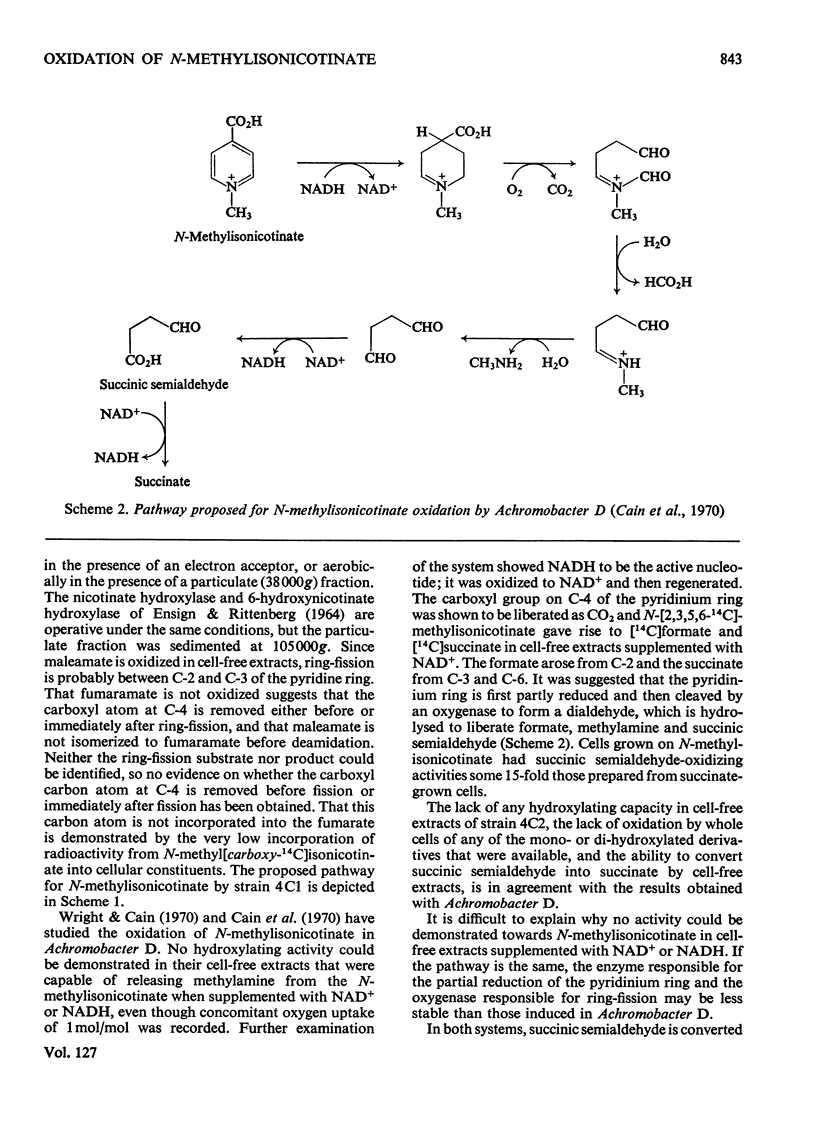
Abstract
Two bacteria have been isolated that are capable of oxidizing N-methylisonicotinate, a photodegradation product of Paraquat (1.1′-dimethyl-4,4′-bipyridylium ion). N-Methylisonicotinate-grown cells of strain 4C1, a Gram-positive rod, oxidized 2-hydroxy-N-methylisonicotinate without lag. Cell-free extracts of these cells converted 2-hydroxyisonicotinate into 2,6-dihydroxyisonicotinate; the reaction did not require molecular oxygen. Maleamate was deamidated and maleate isomerized to fumarate by soluble enzyme systems. [14C]Formaldehyde was isolated as the dimedone derivative from the supernatant of a cell suspension oxidizing N-[14C]methylisonicotinate, and no [14C]-methylamine was detected. Whole cells incubated with N-methyl[carboxy-14C]isonicotinate released 95% of the radioactivity as 14CO2. The second bacterium, strain 4C2, a Gram-negative rod, did not oxidize any of the mono- or di-hydroxypyridines or their N-methyl derivatives that were available or could be synthesized; nor did cell-free extracts oxidize any of these compounds. Methylamine was oxidized by whole cells without lag; cell-free extracts converted methylamine into formaldehyde when a soluble enzyme system requiring an electron acceptor was used; formaldehyde was oxidized to formate and formate to CO2 by enzyme systems requiring NAD+.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBIN D. T. The assay and characterization of amines by 2,4-dinitrofluorobenzene. J Biol Chem. 1960 Mar;235:783–786. [PubMed] [Google Scholar]
- Dutton P. L., Evans W. C. The metabolism of aromatic compounds by Rhodopseudomonas palustris. A new, reductive, method of aromatic ring metabolism. Biochem J. 1969 Jul;113(3):525–536. doi: 10.1042/bj1130525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENSIGN J. C., RITTENBERG S. C. THE PATHWAY OF NICOTINIC ACID OXIDATION BY A BACILLUS SPECIES. J Biol Chem. 1964 Jul;239:2285–2291. [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- Orpin C. G., Knight M., Evans W. C. The bacterial oxidation of picolinamide, a photolytic product of Diquat. Biochem J. 1972 May;127(5):819–831. doi: 10.1042/bj1270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W. Requirement of two protein fractions for O-demethylase activity in Pseudomonas testosteroni. FEBS Lett. 1971 Jan 12;12(3):161–165. doi: 10.1016/0014-5793(71)80058-3. [DOI] [PubMed] [Google Scholar]


