Abstract
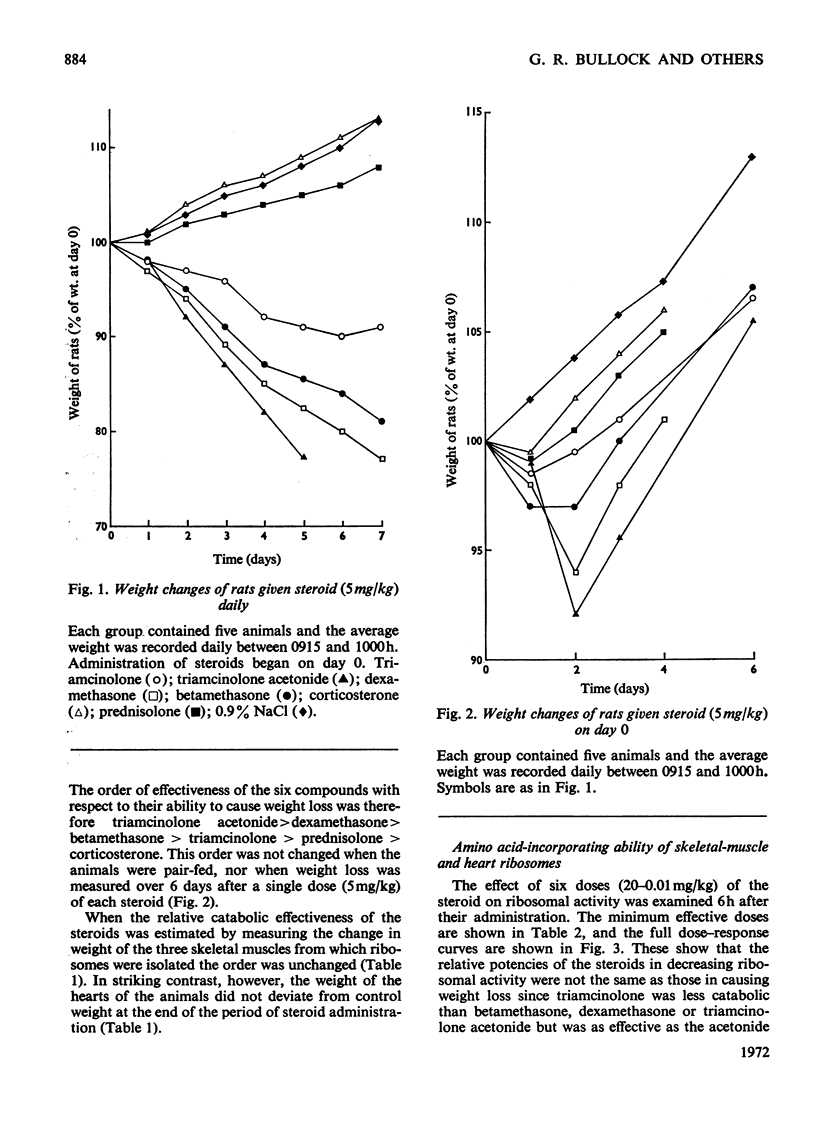
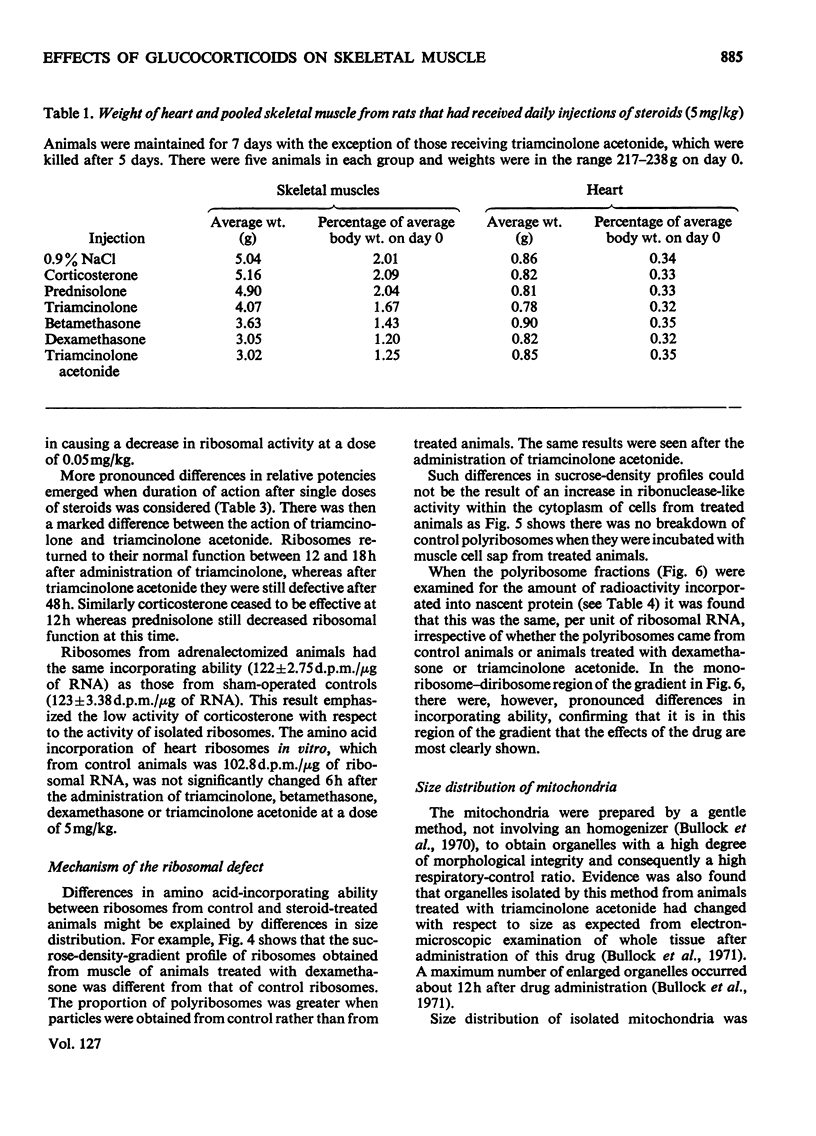
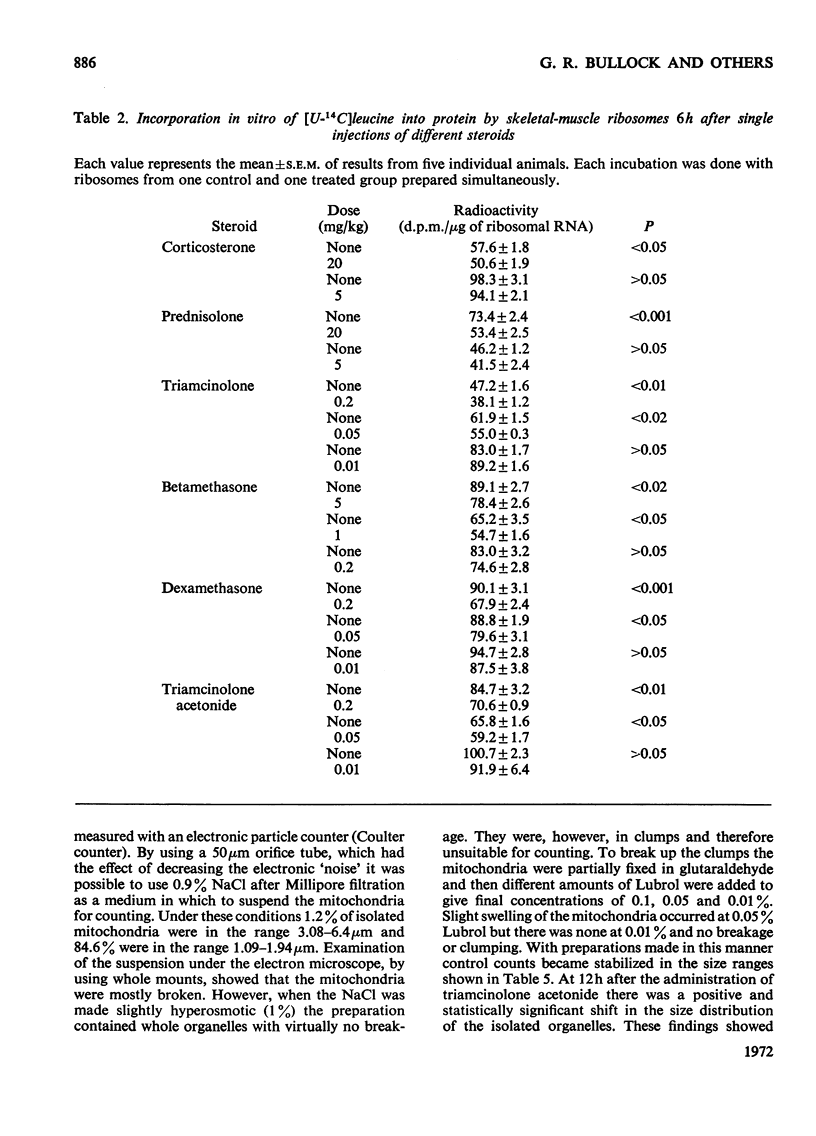
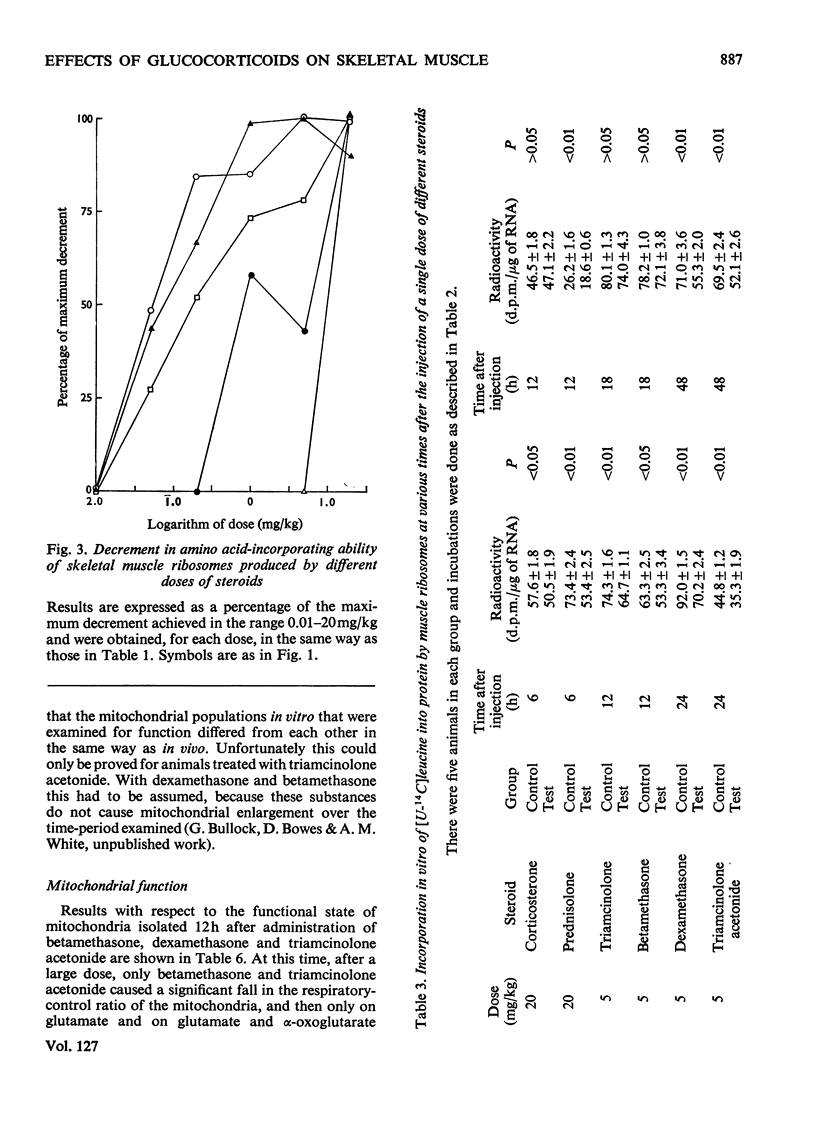
1. Five glucocorticoids, when administered daily to rats for 5–7 days at a dosage of 5mg/kg, were in the following order of effectiveness with respect to their ability to decrease the weight gain of whole animals and the vastus lateralis, vastus medialis and gluteus medius muscles: corticosterone<prednisolone<triamcinolone <betamethasone<dexamethasone<triamcinolone acetonide. 2. The low catabolic potencies of corticosterone and prednisolone were reflected in the high doses (20mg/kg) required to decrease significantly the incorporating activity in vitro of ribosomes isolated from the muscles 6h later. However, triamcinolone was as effective as triamcinolone acetonide in causing a significant effect at 0.05mg/kg, although it had a far lower catabolic potency than the acetonide. 3. The relationship between decrease in ribosomal activity in vitro and weight loss was better understood by considering the duration of the effect after single doses of steroids. Ribosomes returned to normal function between 6 and 12h after the administration of corticosterone (20mg/kg), but still had decreased activity 12h after a similar dose of prednisolone. Normal ribosomal function was renewed between 12 and 18h after the administration of triamcinolone and betamethasone at 5mg/kg, but decreased activity persisted for more than 48h after similar doses of dexamethasone or triamcinolone acetonide. 4. There was no difference in the rate of incorporation of amino acids into nascent protein on polyribosomes from muscle of control animals and animals treated 6h previously with triamcinolone acetonide or dexamethasone. 5. The rate of weight gain of the heart was not affected by any of the steroids tested and heart ribosomes maintained normal activity although concentrations of steroids in this organ 5min after administration were 2–3 times that in skeletal muscle. 6. Mitochondria, isolated from the muscle of animals that had received triamcinolone acetonide (20mg/kg) 12h previously, were shown, by using a Coulter counter, to be enlarged. Nevertheless oxidation was only slightly uncoupled 12h after drug administration and they had normal function 6h later. Similar results with respect to mitochondrial function were obtained after the administration of dexamethasone (20mg/kg) and betamethasone (20mg/kg). 7. From these results it is concluded that the mitochondria are not functionally involved in the early phase of steroid-induced muscle catabolism. The observed decrease in the incorporating ability of muscle ribosomes in vitro appears to be much more closely linked to the initial catabolic event.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- Bullock G. R., Christian R. A., Peters R. F., White A. M. Rapid mitochondrial enlargement in muscle as a response to triamcinolone acetonide and its relationship to the ribosomal defect. Biochem Pharmacol. 1971 May;20(5):943–953. doi: 10.1016/0006-2952(71)90314-5. [DOI] [PubMed] [Google Scholar]
- Bullock G., Carter E. E., White A. M. The preparation of mitochondria from muscle without the use of a homogeniser. FEBS Lett. 1970 May 25;8(2):109–111. doi: 10.1016/0014-5793(70)80237-x. [DOI] [PubMed] [Google Scholar]
- Bullock G., White A. M., Worthington J. The effects of catabolic and anabolic steroids on amino acid incorporation by skeletal-muscle ribosomes. Biochem J. 1968 Jul;108(3):417–425. doi: 10.1042/bj1080417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALUDI G., MILLS L. C., CHAYES Z. W. EFFECT OF STEROIDS ON MUSCLE. Acta Endocrinol (Copenh) 1964 Jan;45:68–78. doi: 10.1530/acta.0.0450068. [DOI] [PubMed] [Google Scholar]
- Hackney J. F., Gross S. R., Aronow L., Pratt W. B. Specific glucocorticoid-binding macromolecules from mouse fibroblasts growing in vitro. A possible steroid receptor for growth inhibition. Mol Pharmacol. 1970 Sep;6(5):500–512. [PubMed] [Google Scholar]
- Krotkiewski M., Krotkiewska J., Björntorp P. Effects of dexamethasone on lipid mobilization in the rat. Acta Endocrinol (Copenh) 1970 Jan;63(1):185–192. doi: 10.1530/acta.0.0630185. [DOI] [PubMed] [Google Scholar]
- Palmer B. G. The effect of cortisol on body weight and muscle metabolism in the rat. J Endocrinol. 1966 Sep;36(1):73–83. doi: 10.1677/joe.0.0360073. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Verhaag D. A., Worsfold M. Studies of steroid myopathy. Examination of the possible effect of triamcinolone on mitochondria and sarcotubular vesicles of rat skeletal muscle. Biochem Pharmacol. 1970 May;19(5):1627–1636. doi: 10.1016/0006-2952(70)90151-6. [DOI] [PubMed] [Google Scholar]
- Peters R. F., Richardson M. C., Small M., White A. M. Some biochemical effects of triamcinolone acetonide on rat liver and muscle. Biochem J. 1970 Feb;116(3):349–355. doi: 10.1042/bj1160349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer M., Weinstein S., Gaylord J., Indindoli L. Rapid procedure for liquid scintillation counting of animal tissues using a nitric acid digestion procedure and a dioxane-based scintillator. Anal Biochem. 1971 Jan;39(1):46–53. doi: 10.1016/0003-2697(71)90459-3. [DOI] [PubMed] [Google Scholar]
- Schaumburg B. P. Studies of the glucocorticoid-binding protein from thymocytes. I. Localization in the cell and some properties of the protein. Biochim Biophys Acta. 1970 Sep 29;214(3):520–532. doi: 10.1016/0005-2795(70)90312-0. [DOI] [PubMed] [Google Scholar]
- TONELLI G., PARTRIDGE R., RINGLER I. BODY AND MUSCLE WEIGHT DEPRESSING EFFECTS AND THYMOLYTIC POTENCIES OF GLUCOCORTICOIDS IN THE RAT. Proc Soc Exp Biol Med. 1965 May;119:136–142. doi: 10.3181/00379727-119-30120. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON E. M., DICKERSON J. W., McCANCE R. A. Severe undernutrition in growing and adult animals. 4. The impact of severe undernutrition on the chemical composition of the soft tissues of the pig. Br J Nutr. 1960;14:457–471. doi: 10.1079/bjn19600060. [DOI] [PubMed] [Google Scholar]
- Wira C., Munck A. Specific glucocorticoid receptors in thymus cells. Localization in the nucleus and extraction of the cortisol-receptor complex. J Biol Chem. 1970 Jul 10;245(13):3436–3438. [PubMed] [Google Scholar]
- Young V. R., Chen S. C., Macdonald J. The sedimentation of rat skeletal-muscle ribosomes. Effect of hydrocortisone, insulin and diet. Biochem J. 1968 Feb;106(4):913–919. doi: 10.1042/bj1060913. [DOI] [PMC free article] [PubMed] [Google Scholar]


