Abstract
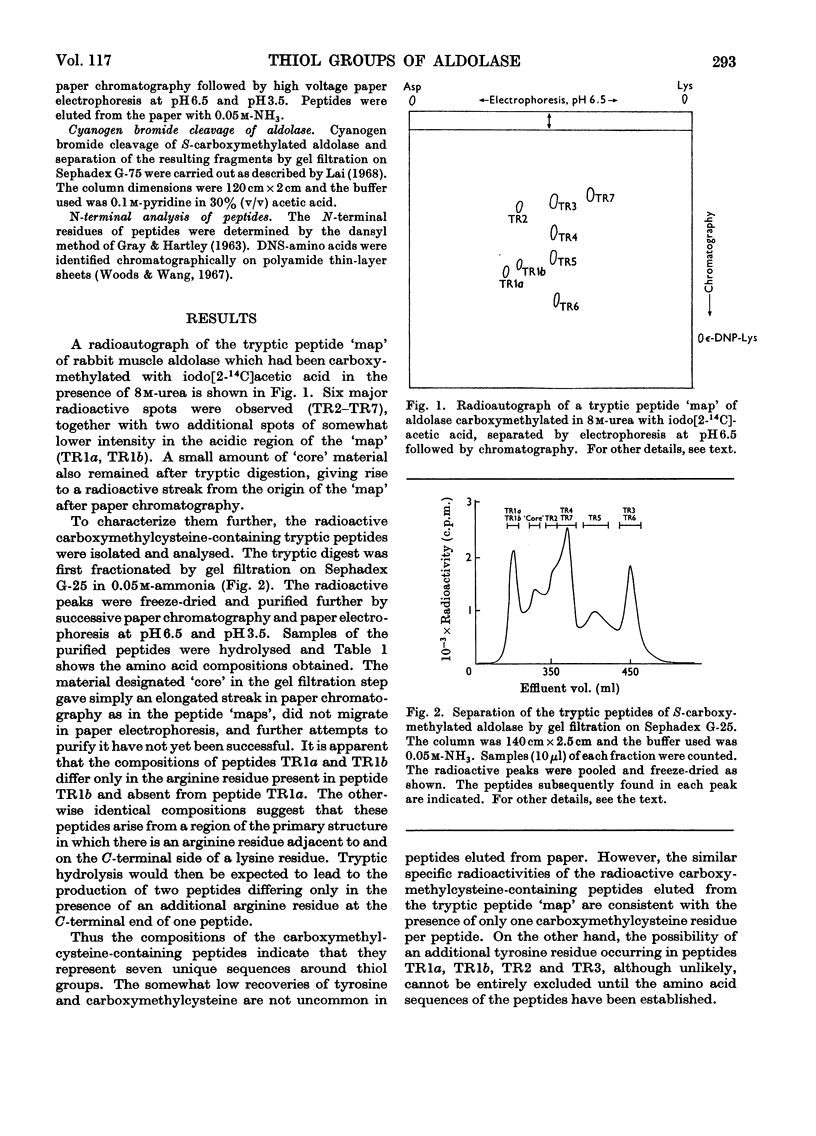
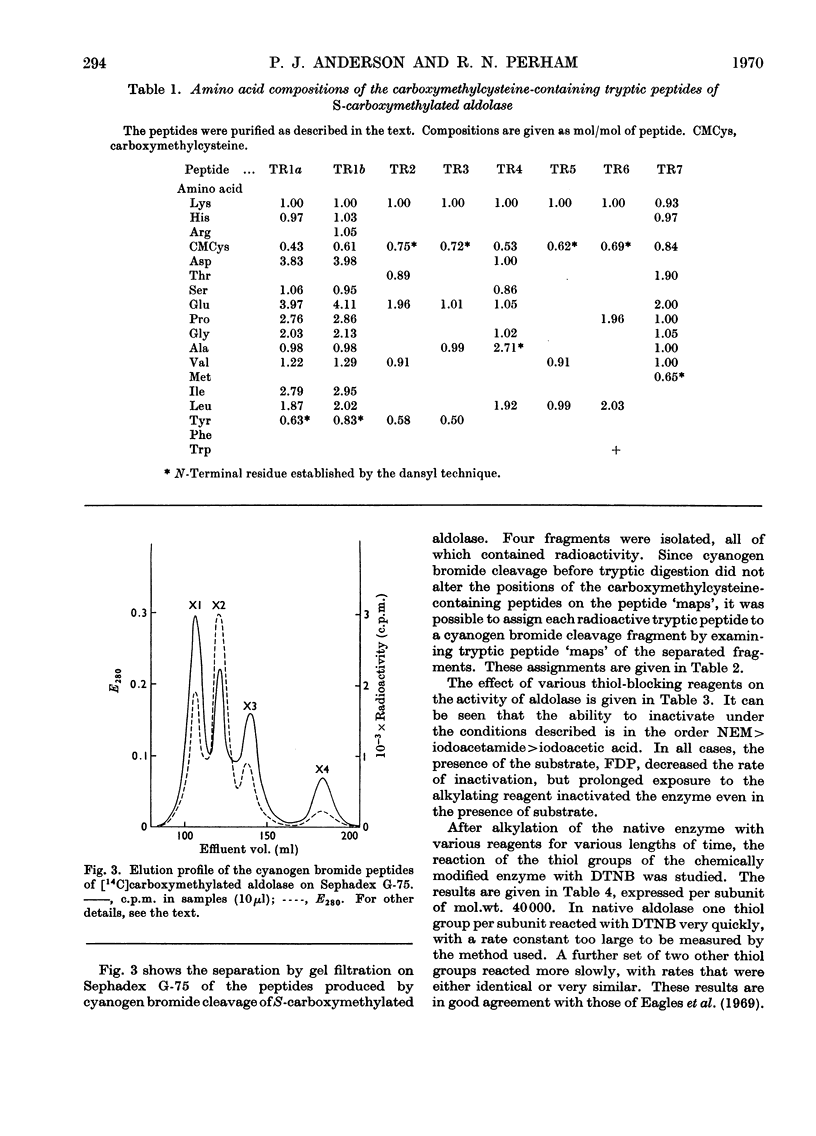
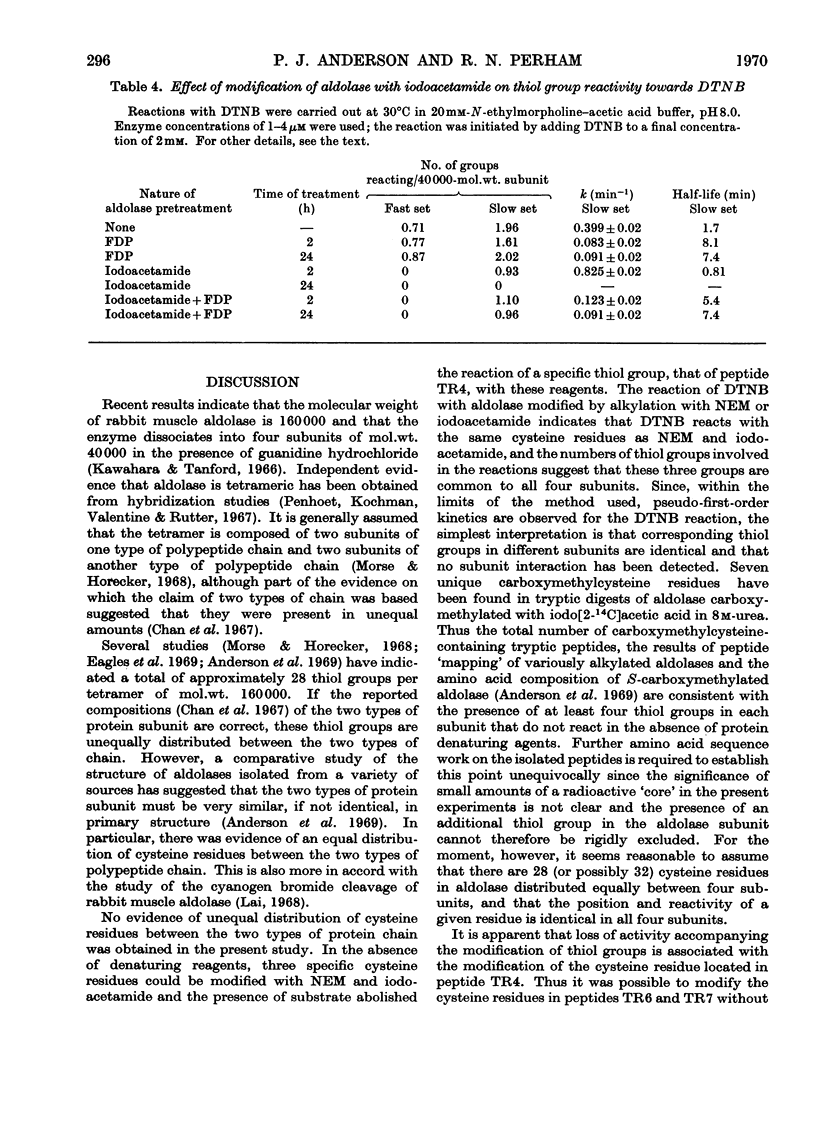
1. Seven unique carboxymethylcysteine-containing peptides have been isolated from tryptic digests of rabbit muscle aldolase carboxymethylated with iodo[2-14C]acetic acid in 8m-urea. These peptides have been characterized by amino acid and end-group analysis and their location within the cyanogen bromide cleavage fragments of the enzyme has been determined. 2. Reaction of native aldolase with 5,5′-dithiobis-(2-nitrobenzoic acid), iodoacetamide and N-ethylmaleimide showed that a total of three cysteine residues per subunit of mol.wt. 40000 were reactive towards these reagents, and that the modification of these residues was accompanied by loss in enzymic activity. Chemical analysis of the modified enzymes demonstrated that the same three thiol groups are involved in the reaction with all these reagents but that the observed reactivity of a given thiol group varies with the reagent used. 3. One reactive thiol group per subunit could be protected when the modification of the enzyme was carried out in the presence of substrate, fructose 1,6-diphosphate, under which conditions enzymic activity was retained. This thiol group has been identified chemically and is possibly at or near the active site. Limiting the exposure of the native enzyme to iodoacetamide also served to restrict alkylation to two thiol groups and left the enzymic activity unimpaired. The thiol group left unmodified is the same as that protected by substrate during more rigorous alkylation, although it is now more reactive towards 5,5′-dithiobis-(2-nitrobenzoic acid) than in the native enzyme. 4. Conversely, prolonged incubation of the enzyme with fructose 1,6-diphosphate, which was subsequently removed by dialysis, caused an irreversible fall in enzymic activity and in thiol group reactivity measured with 5,5′-dithiobis-(2-nitrobenzoic acid). 5. It is concluded that the aldolase tetramer contains at least 28 cysteine residues. Each subunit appears to be identical with respect to number, location and reactivity of thiol groups.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Gibbons I., Perham R. N. A comparative study of the structure of muscle fructose 1,6-diphosphate aldolases. Eur J Biochem. 1969 Dec;11(3):503–509. doi: 10.1111/j.1432-1033.1969.tb00802.x. [DOI] [PubMed] [Google Scholar]
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- BREITENBACH J. W., DERKOSCH J., WESSELY F. Energetics of peptide formation. Nature. 1952 May 31;169(4309):922–922. doi: 10.1038/169922a0. [DOI] [PubMed] [Google Scholar]
- Chan W., Morse D. E., Horecker B. L. Nonidentity of subunits of rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1013–1020. doi: 10.1073/pnas.57.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALGLIESH C. E. The relation between pyridoxin and tryptophan metabolism, studied in the rat. Biochem J. 1952 Sep;52(1):3–14. doi: 10.1042/bj0520003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagles P. A., Johnson L. N., Joynson M. A., McMurray C. H., Gutfreund H. Subunit structure of aldolase: chemical and crystallographic evidence. J Mol Biol. 1969 Nov 14;45(3):533–544. doi: 10.1016/0022-2836(69)90310-6. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- KOWAL J., CREMONA T., HORECKER B. L. THE MECHANISM OF ACTION OF ALDOLASES. IX. NATURE OF THE GROUPS REACTIVE WITH CHLORODINITROBENZENE. J Biol Chem. 1965 Jun;240:2485–2490. [PubMed] [Google Scholar]
- Kawahara K., Tanford C. The number of polypeptide chains in rabbit muscle aldolase. Biochemistry. 1966 May;5(5):1578–1584. doi: 10.1021/bi00869a018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai C. Y., Hoffee P., Horecker B. L. Mechanism of action of aldolases. XII. Primary structure around the substrate binding site of rabbit muscle aldolase. Arch Biochem Biophys. 1965 Dec;112(3):567–579. doi: 10.1016/0003-9861(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Martinez-de Dretz G., Bacila M., Marinello E., Horecker B. L. Labeling of the active site of aldolase with glyceraldehyde 3-phosphate and erythrose 4-phosphate. Biochem Biophys Res Commun. 1968 Mar 27;30(6):665–672. doi: 10.1016/0006-291x(68)90564-0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Horecker B. L. The mechanism of action of aldolases. Adv Enzymol Relat Areas Mol Biol. 1968;31:125–181. doi: 10.1002/9780470122761.ch4. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Kochman M., Valentine R., Rutter W. J. The subunit structure of mammalian fructose diphosphate aldolase. Biochemistry. 1967 Sep;6(9):2940–2949. doi: 10.1021/bi00861a039. [DOI] [PubMed] [Google Scholar]
- Perham R. N. A diagonal paper-electrophoretic technique for studying amino acid sequences around the cysteine and cystine residues of proteins. Biochem J. 1967 Dec;105(3):1203–1207. doi: 10.1042/bj1051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY P. T., TCHOLA O., HORECKER B. L. THE MECHANISM OF ACTION OF ADLOLASES. V. INACTIVATION OF FLUORO- AND CHLORODINITROBENZENE. Arch Biochem Biophys. 1964 Aug;107:305–312. doi: 10.1016/0003-9861(64)90335-2. [DOI] [PubMed] [Google Scholar]
- SZABOLCSI G., BISZKU E. Structural changes induced by the blocking of protein SH groups. Biochim Biophys Acta. 1961 Apr 1;48:335–341. doi: 10.1016/0006-3002(61)90483-8. [DOI] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin B. M. Substrate-induced dissociation of rabbit muscle aldolase into active subunits. Biochem Biophys Res Commun. 1967 Nov 17;29(3):288–293. doi: 10.1016/0006-291x(67)90450-0. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


