Abstract
1. Congenitally goitrous thyroid tissue was obtained from South Australian Merino sheep. Ultrastructural studies of the secretory cells in this tissue showed active cells of normal appearance, containing apical protein droplets. 2. 125I-labelling in vivo of goitre tissue was used to investigate the iodoproteins, in which the major proportion of 125I appeared in the cell protein fraction soluble in 0.9% sodium chloride (average 62% in goitres from untreated sheep). 3. Ammonium sulphate fractionation showed two clear peaks of iodoprotein precipitation, one at 35–40% saturation and the other at 50–55% saturation. Both iodoprotein fractions contained iodotyrosines and iodothyronines, which were identified chromatographically after enzymic hydrolysis of the protein. 4. Polyacrylamide-gel electrophoresis at pH9.4, at either 7.5 or 5.0% acrylamide concentration, was used to characterize the iodoproteins. Two major fractions were observed, the fastest-migrating fraction coincident with serum albumin, and a slower-migrating, less-well-defined zone. This fraction migrated in 7.5% acrylamide gel, which excluded normal thyroglobulin. 5. Density-gradient (10–40% sucrose) centrifugation was used to determine the approximate sedimentation coefficients of the iodoproteins, which showed major components at s20,w 8–9S and s20,w<5S. 6. Immunoprecipitation with rabbit anti-(sheep thyroglobulin) failed to sediment 125I-labelled proteins from goitre extracts. 7. Ouchterlony-type double diffusion in agar plates demonstrated immunoprecipitation lines between rabbit anti-(sheep thyroglobulin) and both the concentrated goitre extract and its Sephadex G-200-excluded fraction, which were confluent with that obtained on reaction with purified normal thyroglobulin. 8. It was concluded that both major iodoprotein fractions were capable of supplying thyroid hormones to the animal, and that the fraction of s20,w<5S was iodinated serum albumin. As 125I-labelled thyroglobulin was not detected in goitre tissue from untreated or thyroxine-treated animals, it was possible that the genetic defect causing goitre resulted in an abnormal thyroglobulin, incapable of being iodinated but immunologically reactive.
Full text
PDF

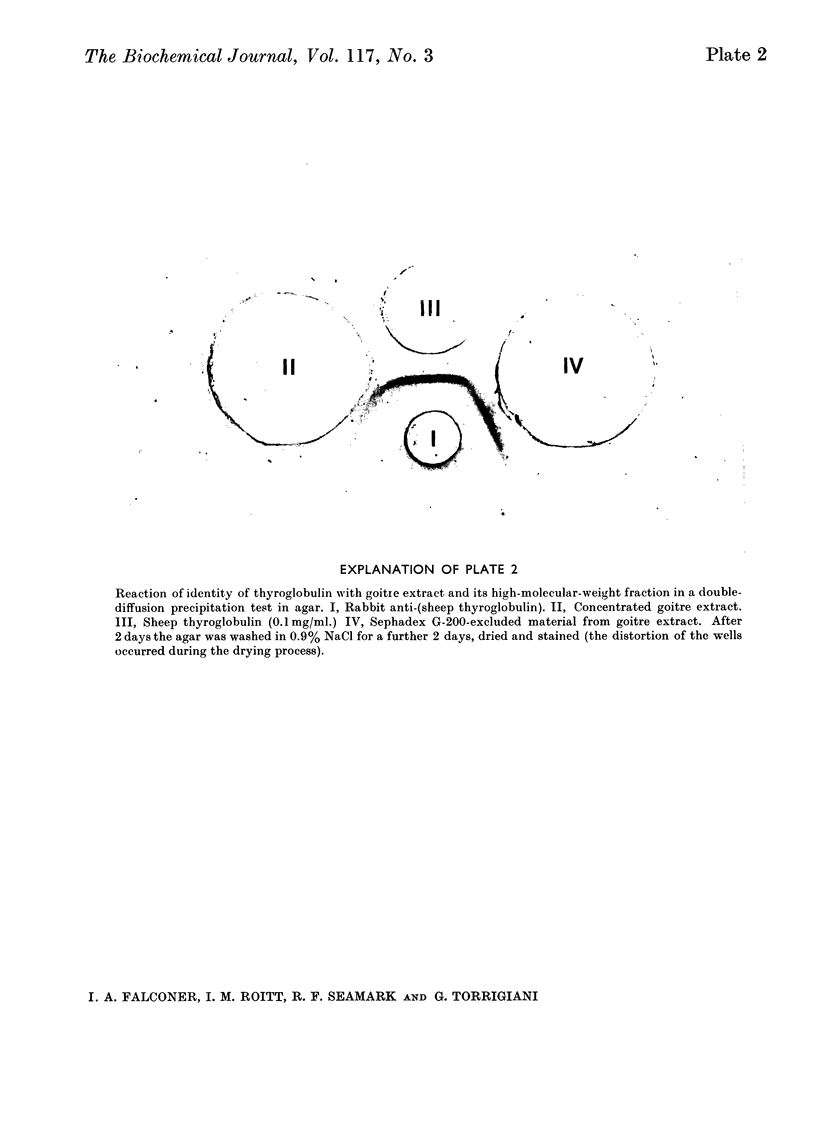
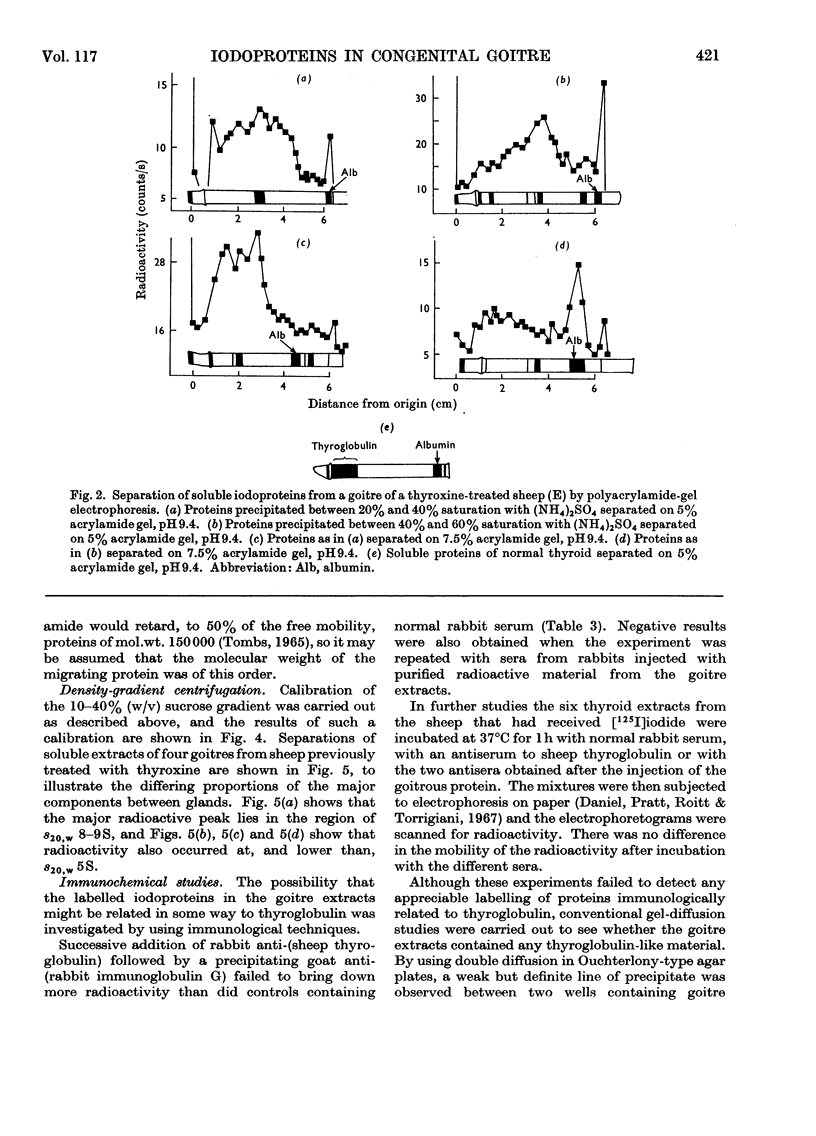
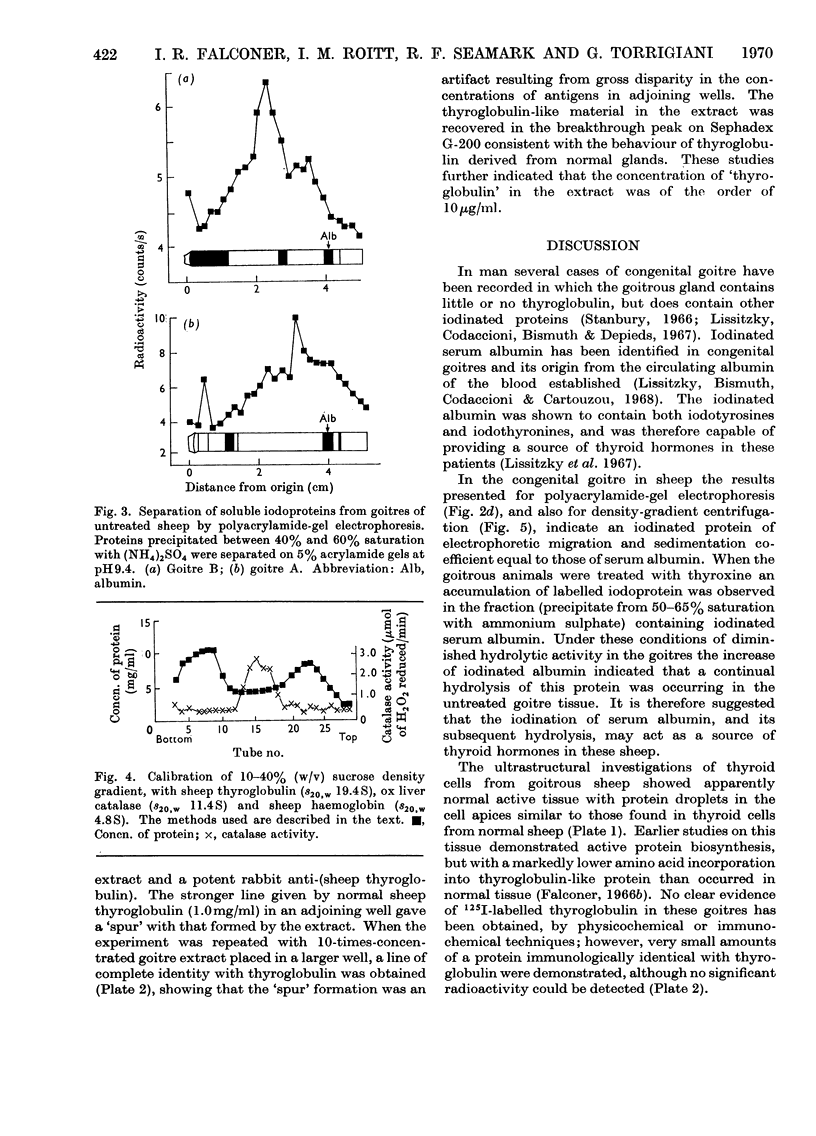
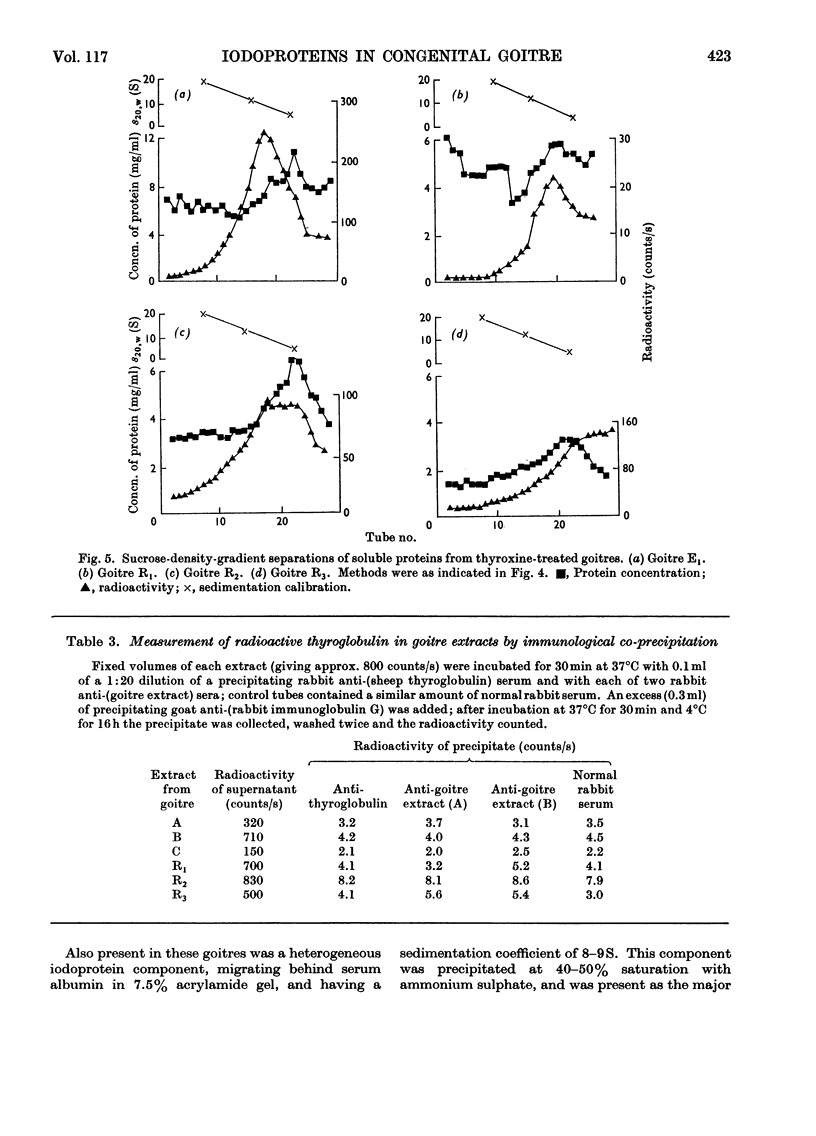

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Pratt O. E., Roitt I. M., Torrigiani G. The release of thyroglobulin from the thyroid gland into thyroid lymphatics; the identification of thyroglobulin in the thyroid lymph and in the blood of monkeys by physical and immunological methods and its estimation by radioimmunoassay. Immunology. 1967 May;12(5):489–504. [PMC free article] [PubMed] [Google Scholar]
- Ekholm R., Smeds S. On dense bodies and droplets in the follicular cells of the guinea pig thyroid. J Ultrastruct Res. 1966 Sep;16(1):71–82. doi: 10.1016/s0022-5320(66)80023-0. [DOI] [PubMed] [Google Scholar]
- Falconer I. R. Studies of the congenitally goitrous sheep. Composition and metabolism of goitrous thyroid tissue. Biochem J. 1966 Jul;100(1):197–203. doi: 10.1042/bj1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer I. R. Studies of the congenitally goitrous sheep. The iodinated compounds of serum, and circulating thyroid-stimulating hormone. Biochem J. 1966 Jul;100(1):190–196. doi: 10.1042/bj1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol. 1956 Nov 25;2(6):799–802. doi: 10.1083/jcb.2.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissitzky S., Bismuth J., Codaccioni J. L., Cartouzou G. Congenital goiter with iodoalbumin replacing thyroglobulin and defect of deiodination of iodotyrosines. Serum origin of the thyroid iodoalbumin. J Clin Endocrinol Metab. 1968 Dec;28(12):1797–1806. doi: 10.1210/jcem-28-12-1797. [DOI] [PubMed] [Google Scholar]
- Lissitzky S., Codaccioni J. L., Bismuth J., Depieds R. Congenital goiter with hypothyroidism and iodo-serum albumin replacing thyroglobulin. J Clin Endocrinol Metab. 1967 Feb;27(2):185–196. doi: 10.1210/jcem-27-2-185. [DOI] [PubMed] [Google Scholar]
- MACLAGAN N. F., BOWDEN C. H., WILKINSON J. H. The metabolism of thyroid hormones. 2. Detection of thyroxine and tri-iodothyronine in human plasma. Biochem J. 1957 Sep;67(1):5–11. doi: 10.1042/bj0670005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rac R., Hill G. N., Pain R. W., Wulhearn C. J. Congenital goitre in Merino sheep due to an inherited defect in the biosynthesis of thyroid hormone. Res Vet Sci. 1968 May;9(3):209–223. [PubMed] [Google Scholar]
- Robbins J., Van Zyl A., Van der Walt K. Abnormal thyroglobin in congenital goiter of cattle. Endocrinology. 1966 Jun;78(6):1213–1223. doi: 10.1210/endo-78-6-1213. [DOI] [PubMed] [Google Scholar]
- SAMEJIMA T., YANG J. T. RECONSTITUTION OF ACID-DENATURED CATALASE. J Biol Chem. 1963 Oct;238:3256–3261. [PubMed] [Google Scholar]
- Triantaphyllidis E., Thompson B. D., Barnaby C. F., Jasani B. Iodination of serum proteins in vivo: relationship to irradiation and hypertrophy of the thyroid. J Endocrinol. 1969 Aug;44(4):467–474. doi: 10.1677/joe.0.0440467. [DOI] [PubMed] [Google Scholar]