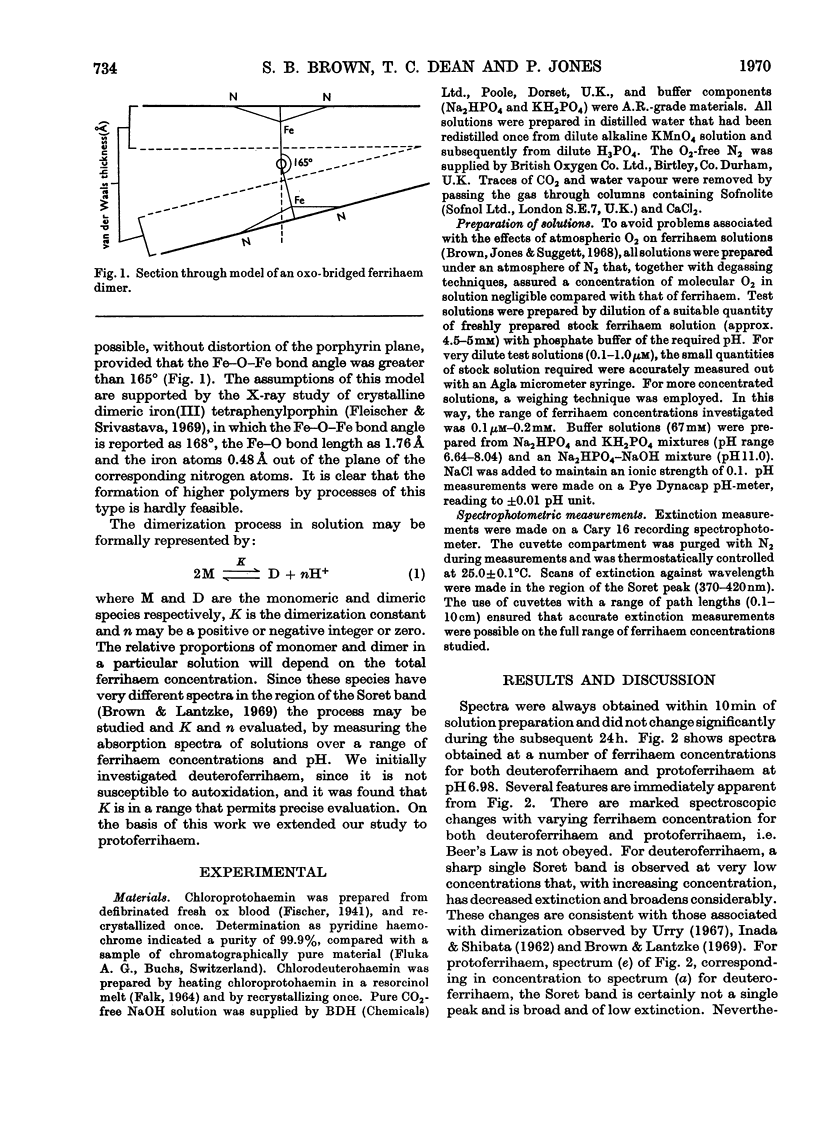
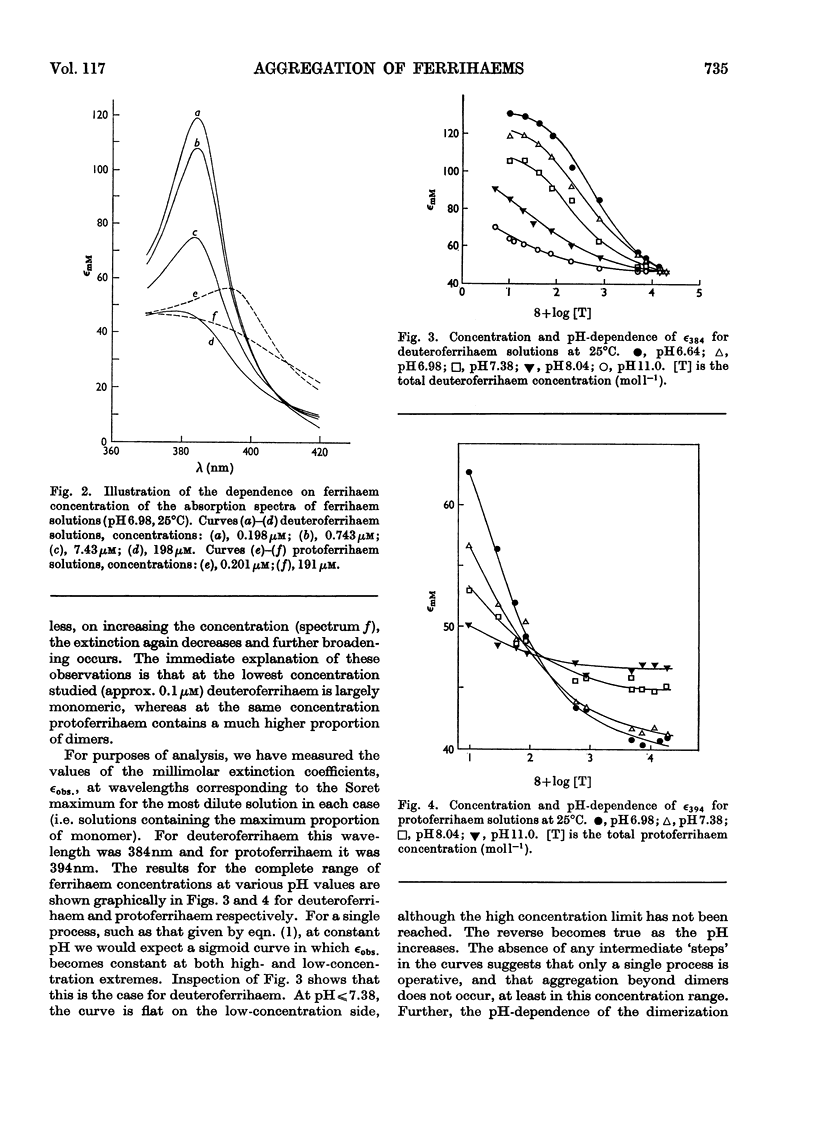
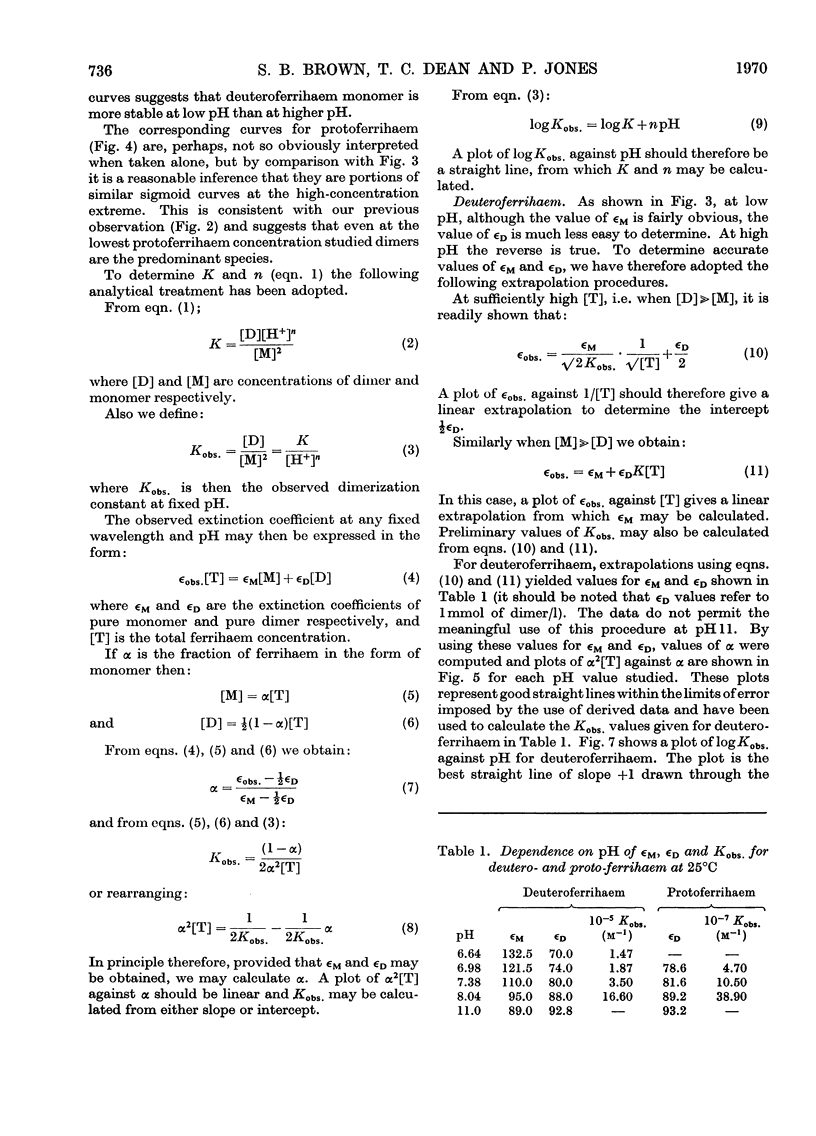
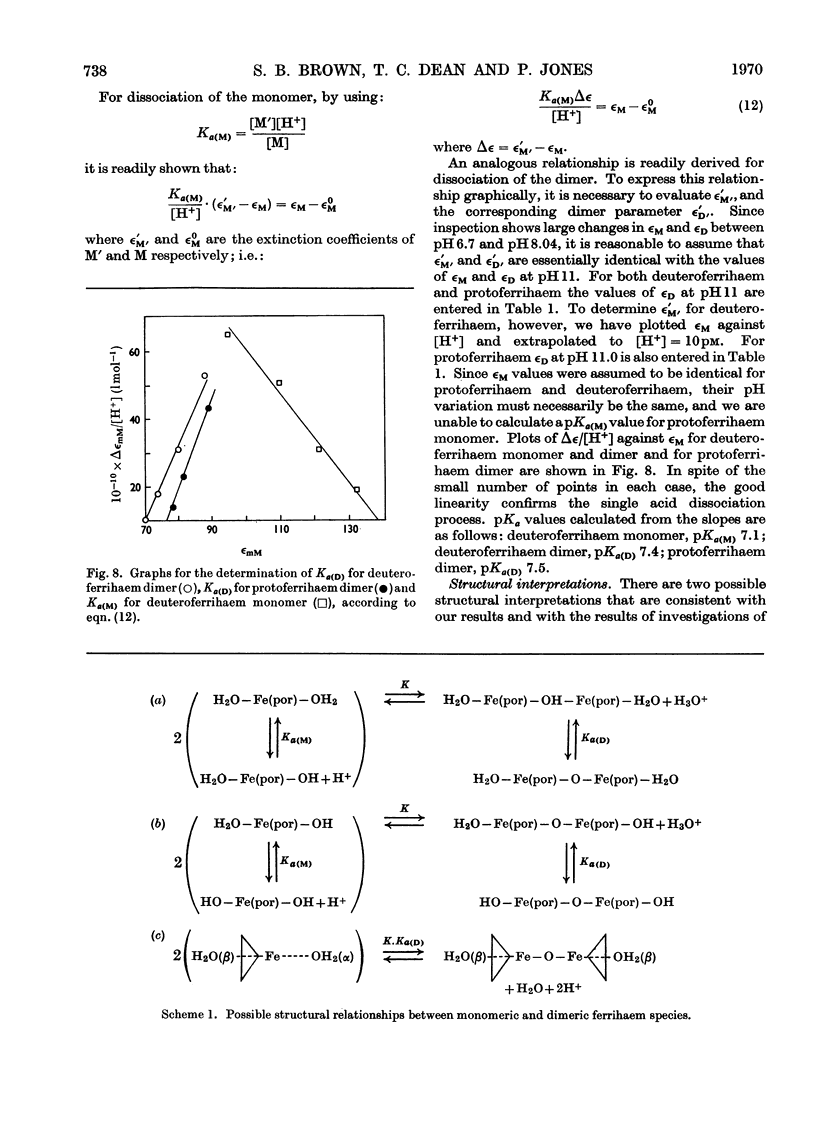
Abstract
1. The absorption spectra of deutero- and proto-ferrihaem in aqueous solution at 25°C show marked changes with concentration and pH in the Soret band region. Quantitative studies of these phenomena imply that they are associated with ferrihaem dimerization and with protolytic equilibria involving monomeric (M) and dimeric (D) ferrihaem species according to the scheme: [Formula: see text] 2. For deuteroferrihaem we obtain K=1.9×10−2, pKa(M)=7.1, pKa(D)=7.4. Protoferrihaem has a much higher dimerization constant, K=4.5 and pKa(D)=7.5 (pKa(M) is not accessible). 3. Possible structural relationships between monomeric and dimeric ferrihaem species in solution are discussed in relation to recent work on the oxo-bridged nature of crystalline ferrihaem dimers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. B., Jones P., Lantzke I. R. Infrared evidence for an oxo-bridged (Fe-O-Fe) haemin dimer. Nature. 1969 Aug 30;223(5209):960–961. doi: 10.1038/223960a0. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Lantzke I. R. Solution structures of ferrihaem in some dipolar aprotic solvents and their binary aqueous mixtures. Biochem J. 1969 Nov;115(2):279–285. doi: 10.1042/bj1150279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWGILL R. W., CLARK W. M. Metalloporphyrins. VII. Coordination of imidazoles with ferrimesoporphyrin. J Biol Chem. 1952 Sep;198(1):33–61. [PubMed] [Google Scholar]
- INADA Y., SHIBATA K. The Soret band of monomeric hematin and its changes on polymerization. Biochem Biophys Res Commun. 1962 Oct 31;9:323–327. doi: 10.1016/0006-291x(62)90048-7. [DOI] [PubMed] [Google Scholar]
- KOENIG D. F. THE STRUCTURE OF ALPHA-CHLOROHEMIN. Acta Crystallogr. 1965 Apr 10;18:663–673. doi: 10.1107/s0365110x65001536. [DOI] [PubMed] [Google Scholar]
- Leipert T. K., Baldeschwieler J. D., Shirley D. A. Applications of gamma ray angular correlations to the study of biological macromolecules in solution. Nature. 1968 Nov 30;220(5170):907–909. doi: 10.1038/220907a0. [DOI] [PubMed] [Google Scholar]
- ROTHSCHILD M. L. The reaction of ferriprotoporphyrin with hydrogen peroxide in alkaline solutions. Arch Biochem Biophys. 1960 Oct;90:229–233. doi: 10.1016/0003-9861(60)90572-5. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Model systems for interacting heme moieties. I. The heme undecapeptide of cytochrome c. J Am Chem Soc. 1967 Aug 2;89(16):4190–4196. doi: 10.1021/ja00992a601. [DOI] [PubMed] [Google Scholar]


