Abstract
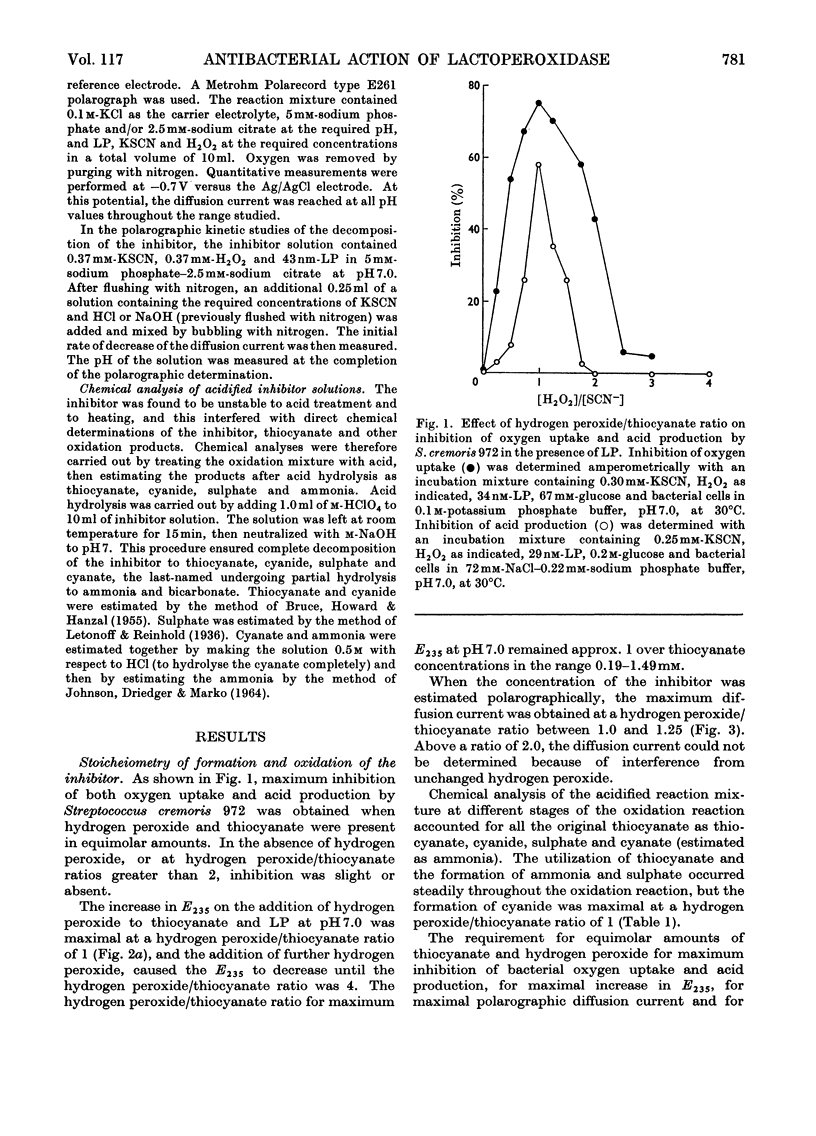
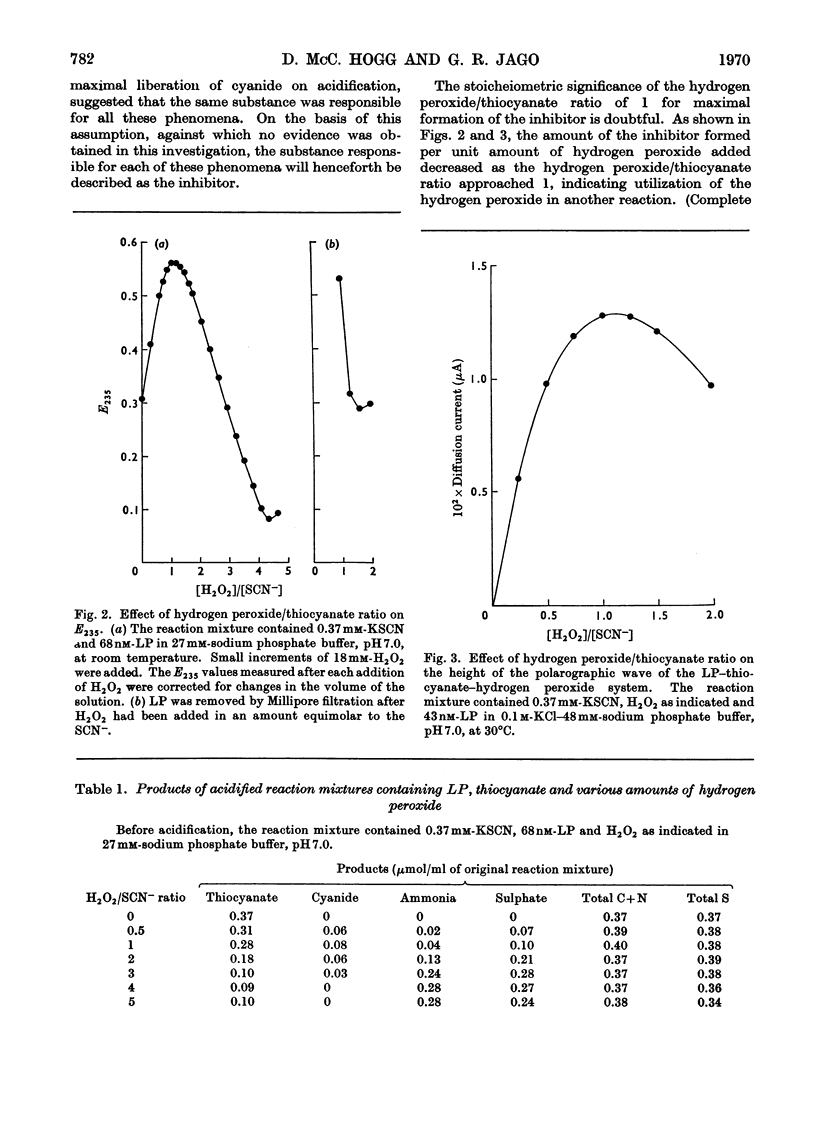
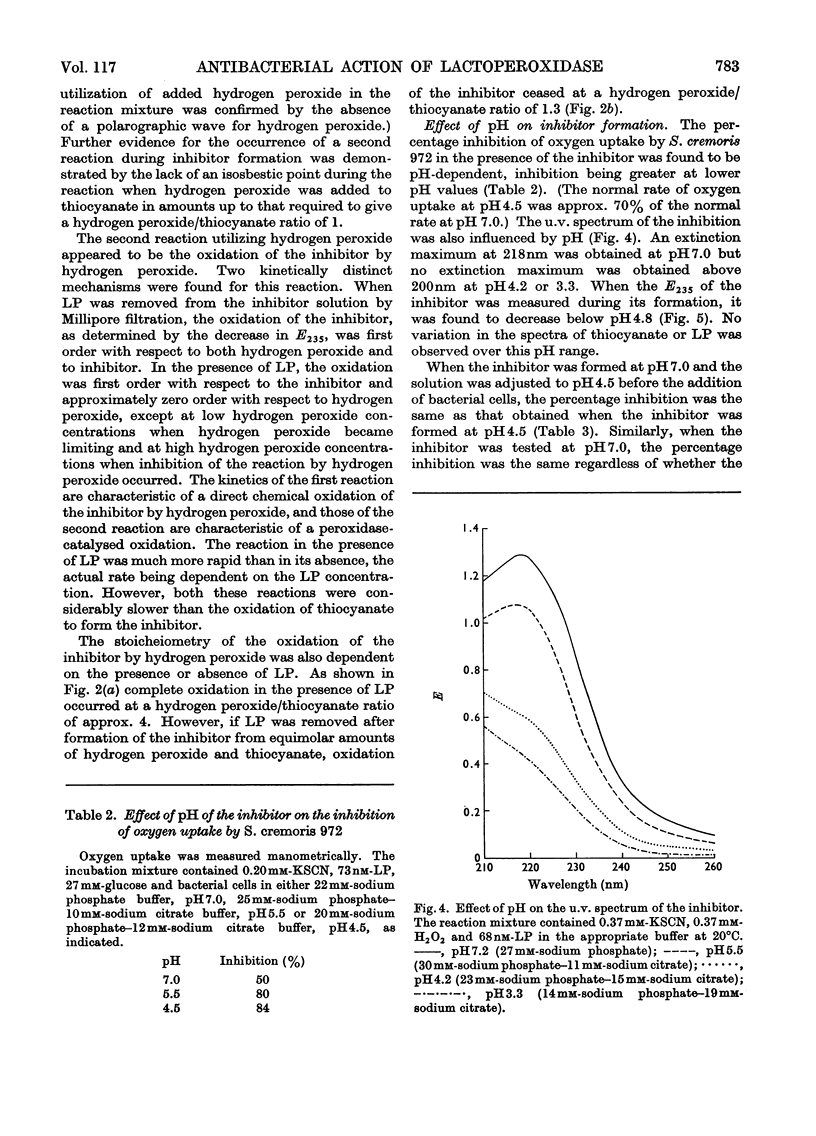
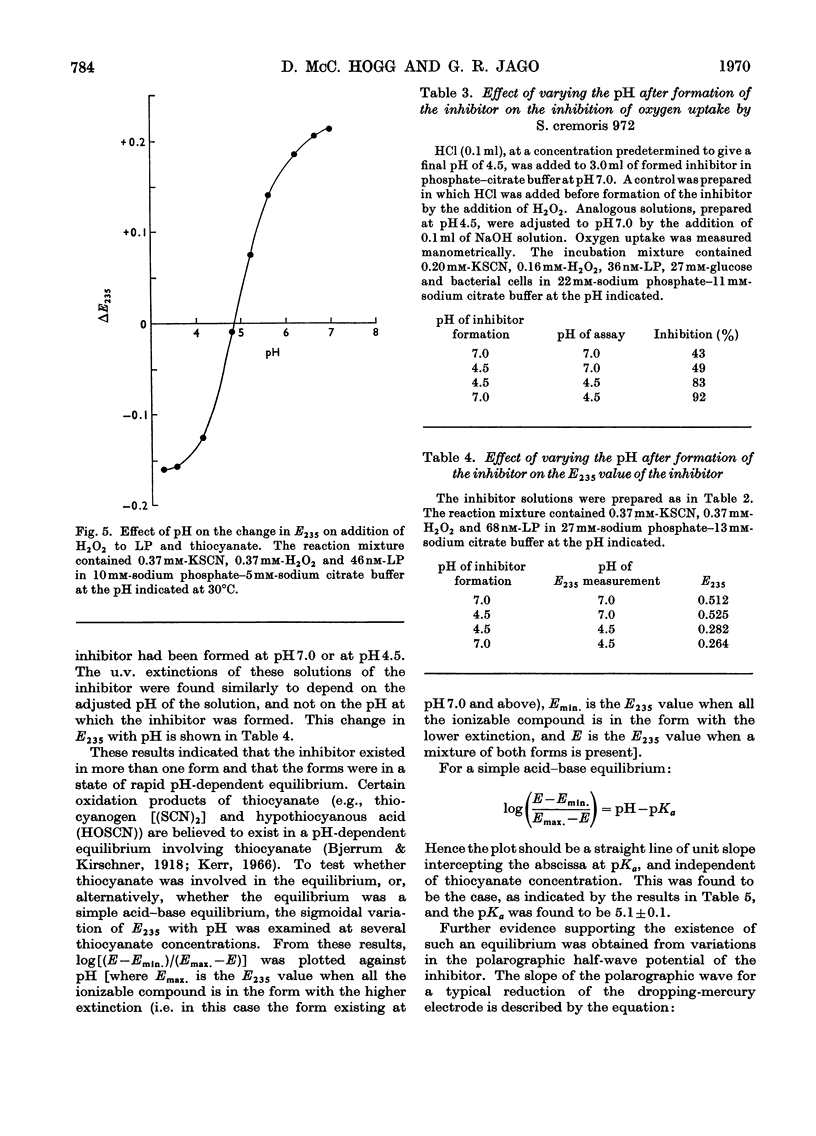
Lactoperoxidase (EC 1.11.1.7), an enzyme present in various mammalian glands and in their secretions, catalyses the oxidation of thiocyanate by hydrogen peroxide to form a compound that inhibits the growth, oxygen uptake and acid production of certain bacteria. This compound was found to be too unstable to isolate in pure form, but its properties in dilute aqueous solution were studied with a view to establishing its identity. At thiocyanate concentrations of approximately 1mm, formation of the inhibitor, which took place by a nonstoicheiometric reaction, was maximal when an approximately equimolar amount of hydrogen peroxide was added. Excess of hydrogen peroxide oxidized the inhibitor to sulphate and cyanate. The inhibitor displayed a polarographic reduction wave of which the half-wave potential was pH-dependent. Studies of the variation of the polarographic half-wave potential and of the u.v. extinction with pH indicated that the inhibitor existed in an acid–base equilibrium (pKa 5.1±0.1). The inhibitor decomposed by a mechanism involving H+ ions and thiocyanate, the kinetics varying according to whether the inhibitor was in its acidic or basic form. From these studies it was concluded that the inhibitor was either cyanosulphurous acid (HO2SCN) or cyanosulphuric acid (HO3SCN).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clem W. H., Klebanoff S. J. Inhibitory effect of saliva on glutamic acid accumulation by Lactobacillus acidophilus and the role of the lactoperoxidase-thiocyanate system. J Bacteriol. 1966 May;91(5):1848–1853. doi: 10.1128/jb.91.5.1848-1853.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGO G. R., MORRISON M. Anti-streptococcal activity of lactoperoxidase III. Proc Soc Exp Biol Med. 1962 Dec;111:585–588. doi: 10.3181/00379727-111-27862. [DOI] [PubMed] [Google Scholar]
- JOHNSON L. D., DRIEDGER A., MARKO A. M. CHROMATOGRAPHIC BEHAVOR OF HISTONES ON SEPHADEX AND CARBOXYMETHYLCELLULOSE. Can J Biochem. 1964 Jun;42:795–811. doi: 10.1139/o64-091. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J., LUEBKE R. G. THE ANTILACTOBACILLUS SYSTEM OF SALIVA. ROLE OF SALIVARY PEROXIDASE. Proc Soc Exp Biol Med. 1965 Feb;118:483–486. doi: 10.3181/00379727-118-29882. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HAMILTON H. B., STOTZ E. The isolation and purification of lactoperoxidase by ion exchange chromatography. J Biol Chem. 1957 Oct;228(2):767–776. [PubMed] [Google Scholar]
- Mickelson M. N. Effect of lactoperoxidase and thiocyanate on the growth of Streptococcus pyogenes and Streptococcus agalactiae in a chemically defined culture medium. J Gen Microbiol. 1966 Apr;43(1):31–43. doi: 10.1099/00221287-43-1-31. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J. 1966 Aug;100(2):373–381. doi: 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The oxidation of thiocyanate and the nature of the inhibitory compound. Biochem J. 1966 Aug;100(2):382–388. doi: 10.1042/bj1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowey R. R., Eidelman S., Klebanoff S. J. Antibacterial activity of the purified peroxidase from human parotid saliva. J Bacteriol. 1968 Sep;96(3):575–579. doi: 10.1128/jb.96.3.575-579.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele W. F., Morrison M. Antistreptococcal activity of lactoperoxidase. J Bacteriol. 1969 Feb;97(2):635–639. doi: 10.1128/jb.97.2.635-639.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


