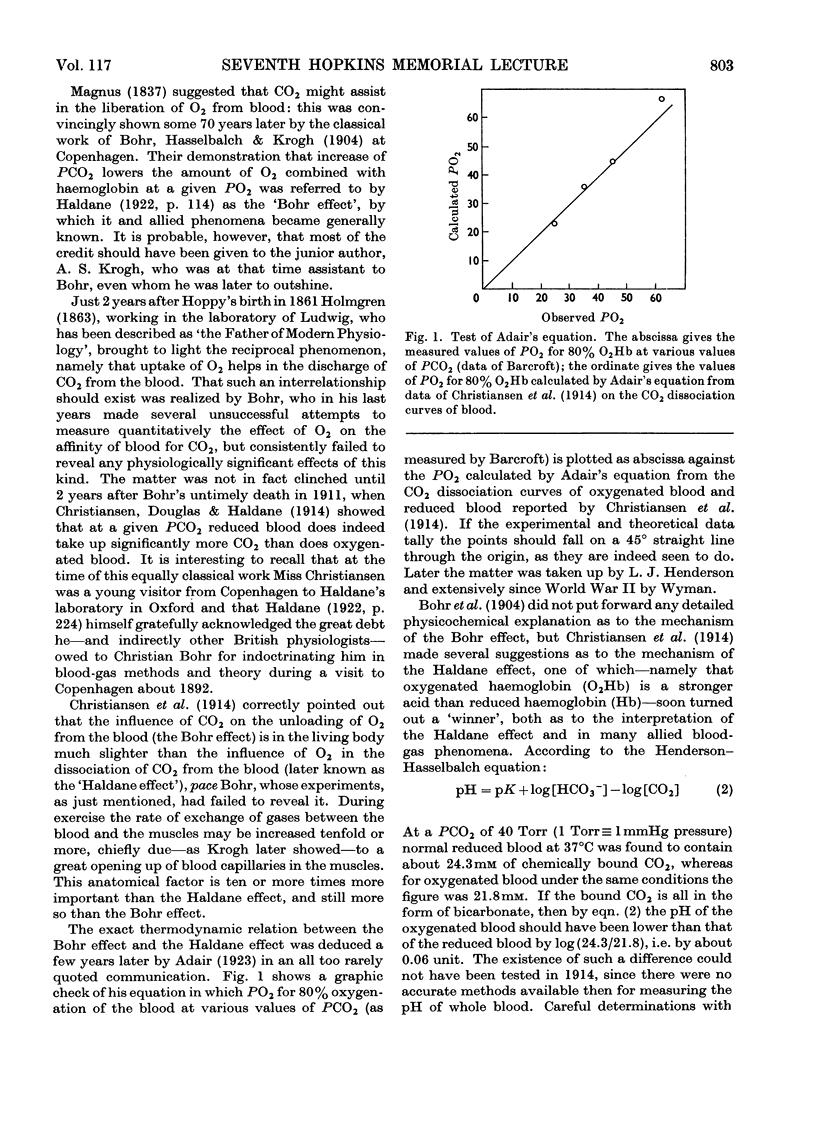
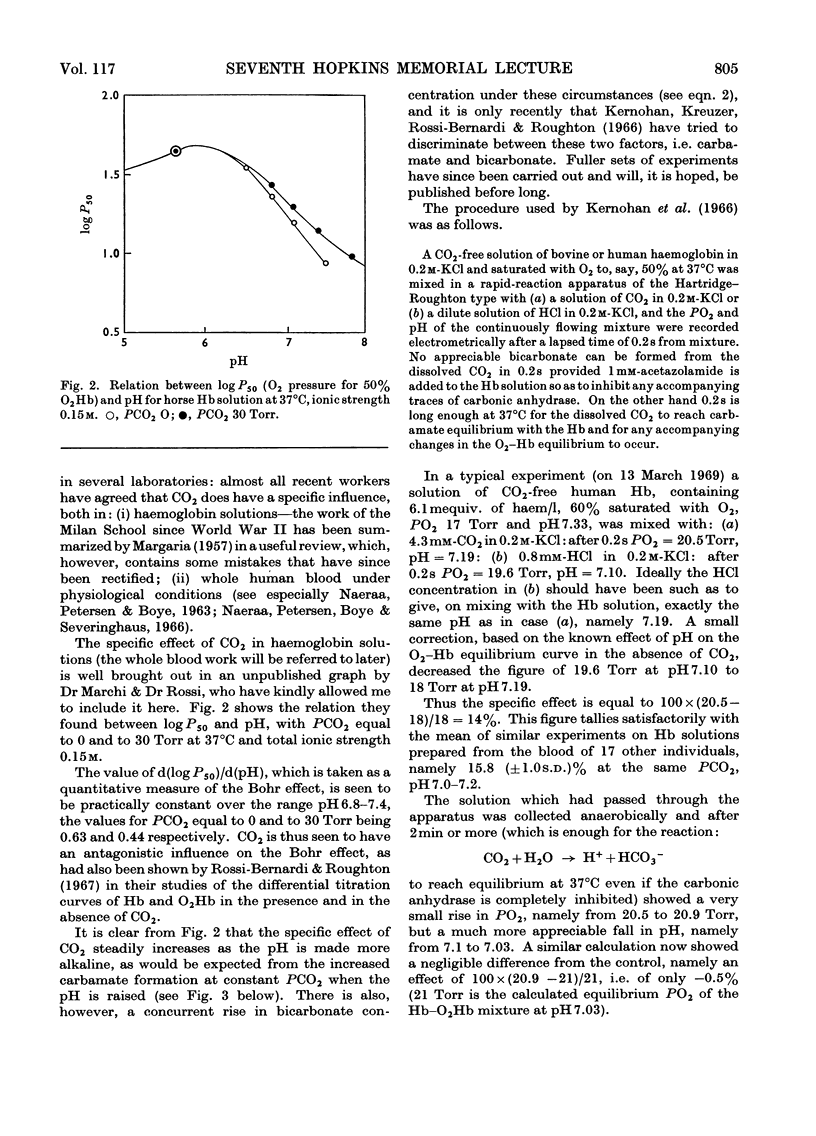
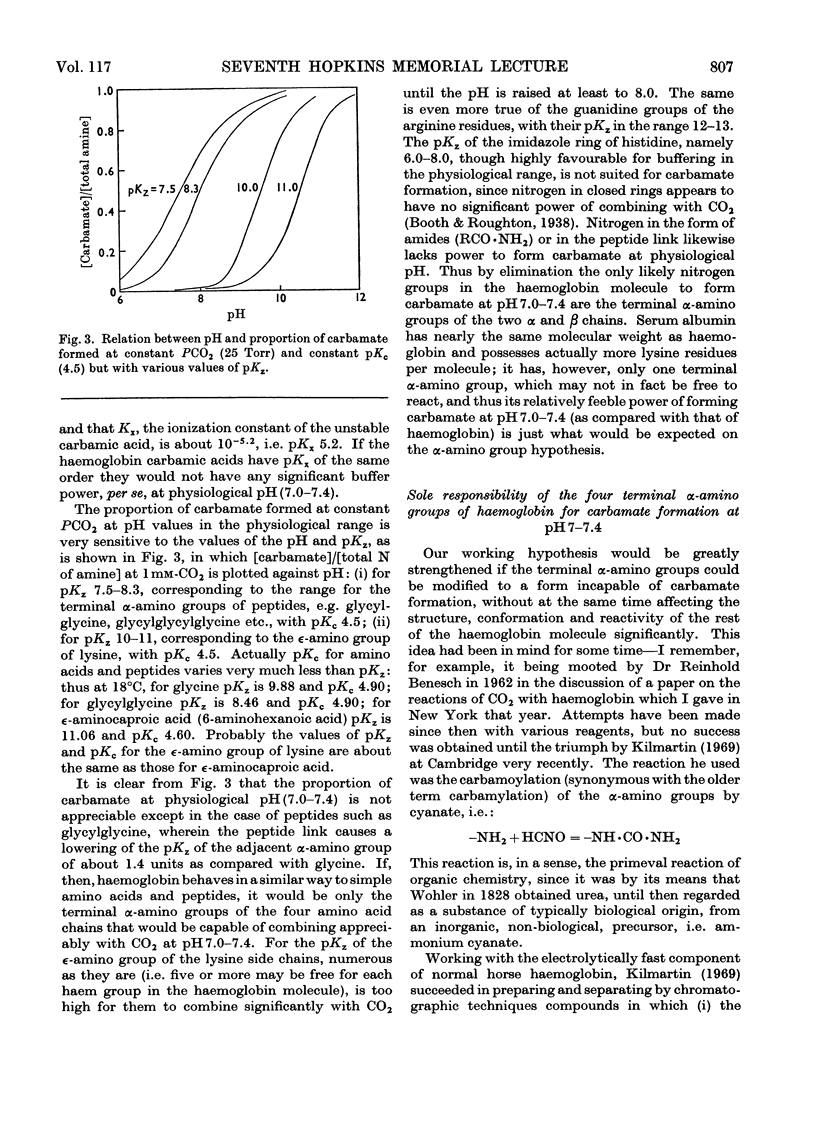
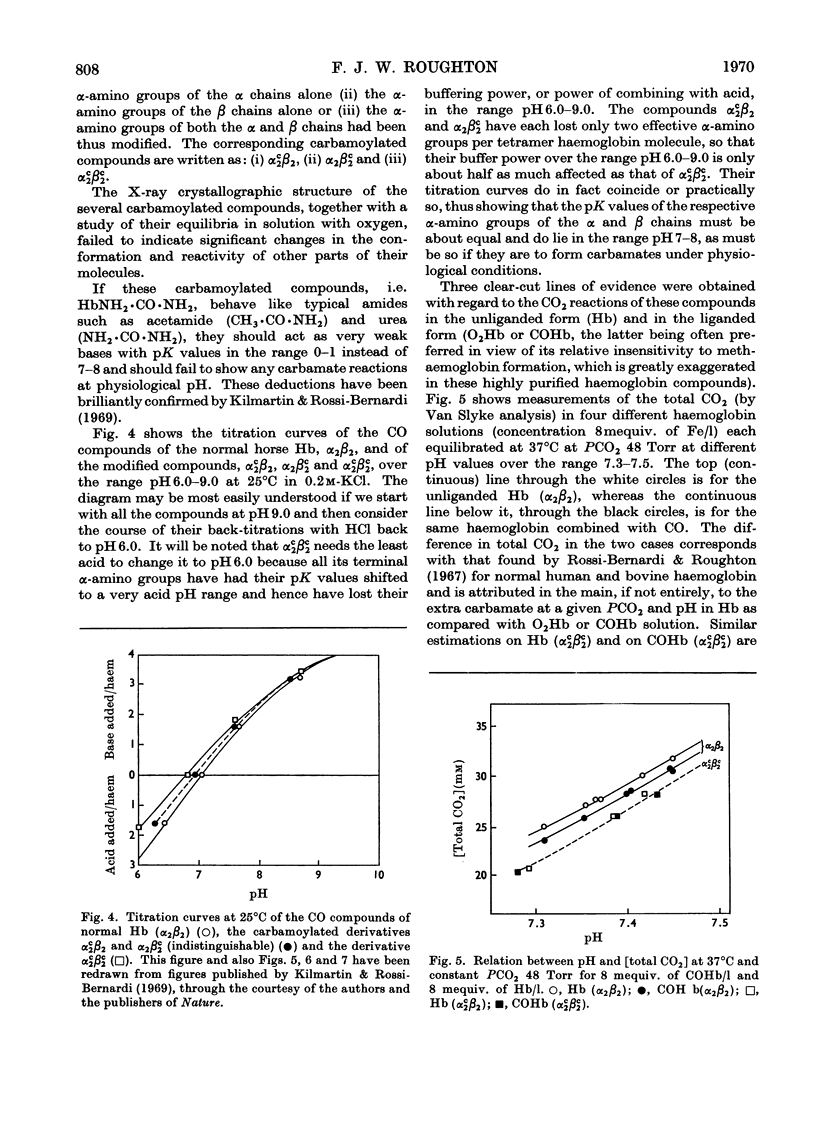
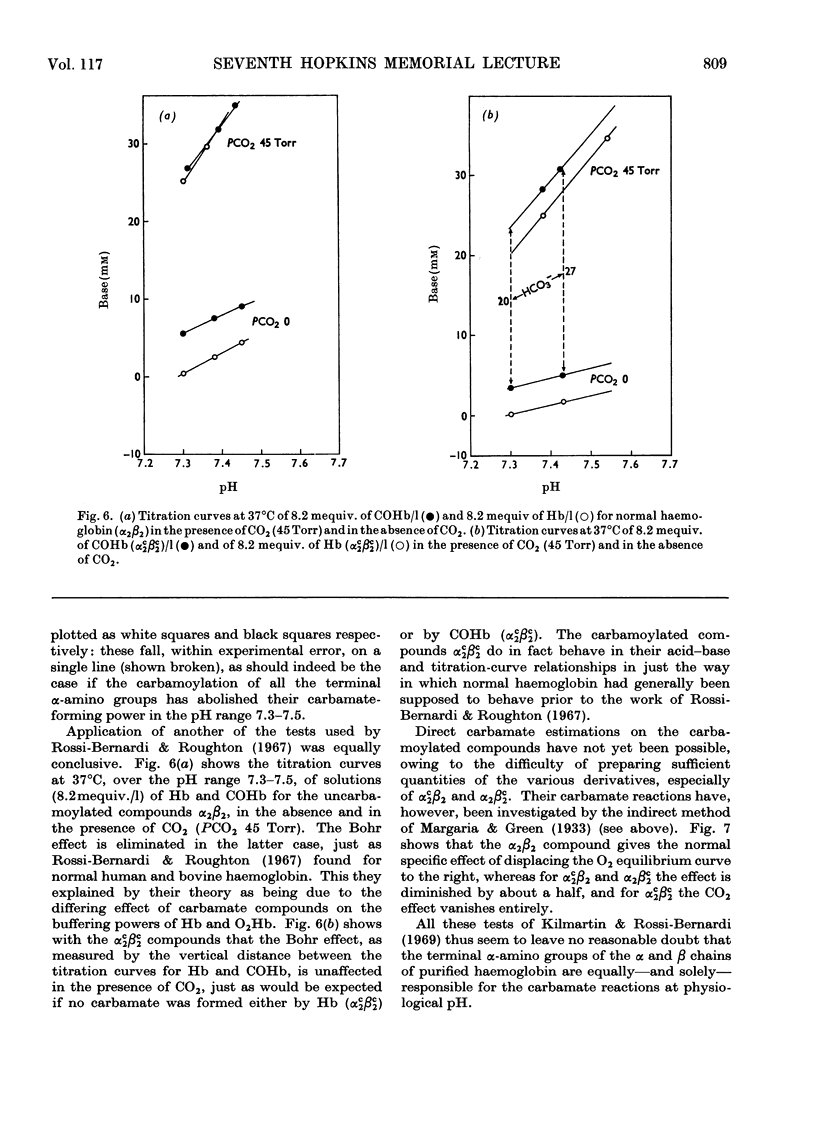
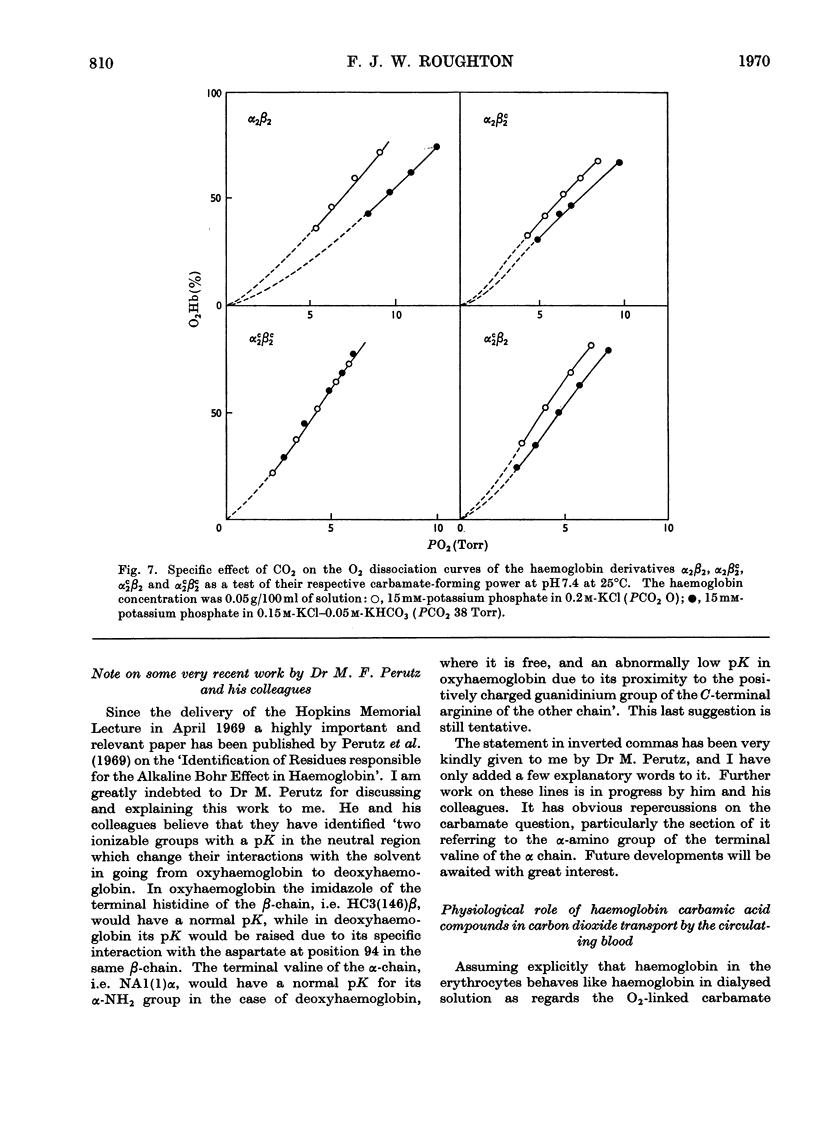
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. Antagonistic influence of Co2 and 2,3 diphosphoglycerate on the Bohr effect of human haemoglobin. Life Sci. 1969 Oct 15;8(20):1041–1046. doi: 10.1016/0024-3205(69)90455-x. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- CONSTANTINE H. P., CRAW M. R., FORSTER R. E. RATE OF THE REACTION OF CARBON DIOXIDE WITH HUMAN RED BLOOD CELLS. Am J Physiol. 1965 Apr;208:801–811. doi: 10.1152/ajplegacy.1965.208.4.801. [DOI] [PubMed] [Google Scholar]
- Chipperfield J. R. The kinetics of combination of carbon dioxide with the anions of glycine, glycyl-glycine, and related amino acids. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):401–410. doi: 10.1098/rspb.1966.0040. [DOI] [PubMed] [Google Scholar]
- Christiansen J., Douglas C. G., Haldane J. S. The absorption and dissociation of carbon dioxide by human blood. J Physiol. 1914 Jul 14;48(4):244–271. doi: 10.1113/jphysiol.1914.sp001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem J. 1940 Sep;34(8-9):1163–1176. doi: 10.1042/bj0341163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernohan J. C., Roughton F. J. Thermal studies of the rates of the reactions of carbon dioxide in concentrated haemoglobin solutions and in red blood cells. A. The reactions catalysed by carbonic anhydrase. B. The carbamino reactions of oxygenated and deoxygenated haemoglobin. J Physiol. 1968 Jul;197(2):345–361. doi: 10.1113/jphysiol.1968.sp008563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature. 1969 Jun 28;222(5200):1243–1246. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- MARGARIA R. The contribution of hemoglobin to acid-base equilibrium of the blood in health and disease. Clin Chem. 1957 Aug;3(4 Pt 2):306–318. [PubMed] [Google Scholar]
- Meldrum N. U., Roughton F. J. The state of carbon dioxide in blood. J Physiol. 1933 Dec 5;80(2):143–170. doi: 10.1113/jphysiol.1933.sp003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAERAA N., PETERSEN E. S., BOYE E. The influence of simultaneous, independent changes in pH and carbon dioxide tension on the in vitro oxygen tension-saturation relationship of human blood. Scand J Clin Lab Invest. 1963;15:141–151. doi: 10.1080/00365516309051325. [DOI] [PubMed] [Google Scholar]
- Naeraa N., Petersen E. S., Boye E., Severinghaus J. W. pH and molecular CO2 components of the Bohr effect in human blood. Scand J Clin Lab Invest. 1966;18(1):96–102. doi: 10.3109/00365516609065612. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Mazzarella L., Crowther R. A., Greer J., Kilmartin J. V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969 Jun 28;222(5200):1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- Roughton F. J., Booth V. H. The catalytic effect of buffers on the reaction CO(2)+H(2)Oright harpoon over left harpoonH(2)CO(3). Biochem J. 1938 Nov;32(11):2049–2069. doi: 10.1042/bj0322049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton F. J., Rossi-Bernardi L. The carbamate reaction of carbon dioxide with glycyl-glycine. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):381–400. doi: 10.1098/rspb.1966.0039. [DOI] [PubMed] [Google Scholar]



