Abstract
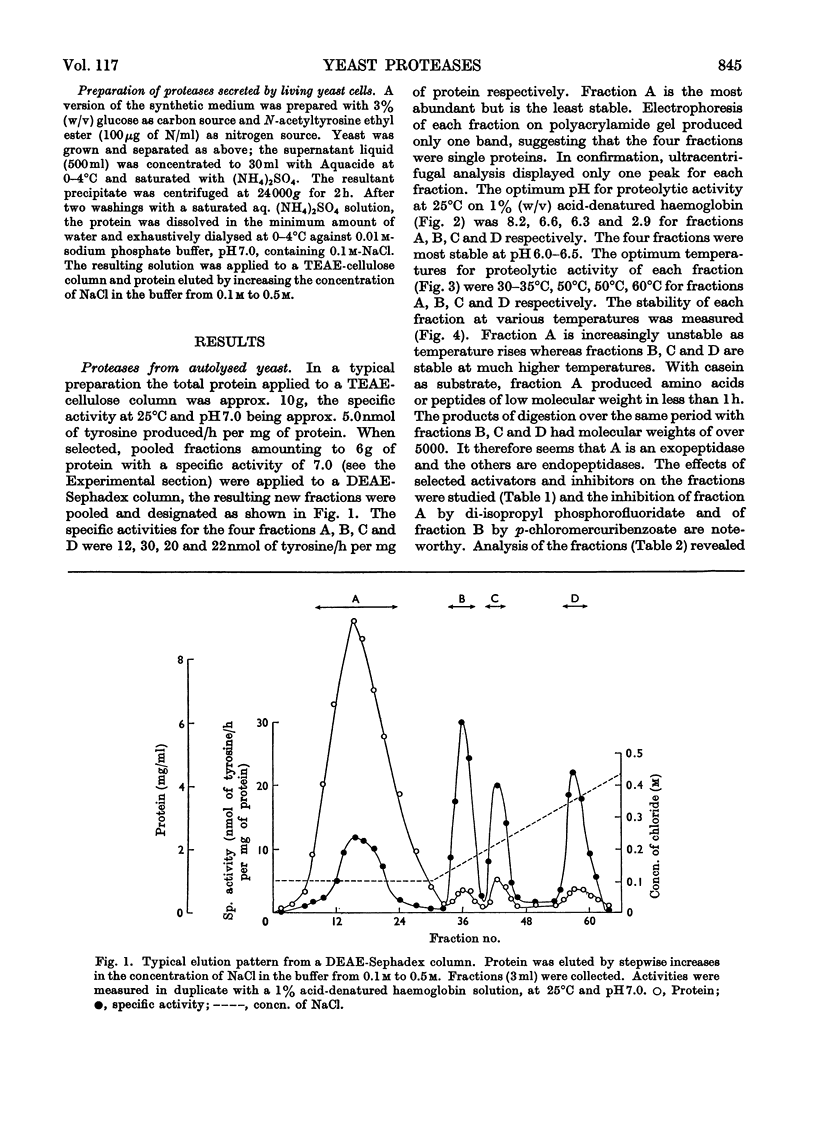
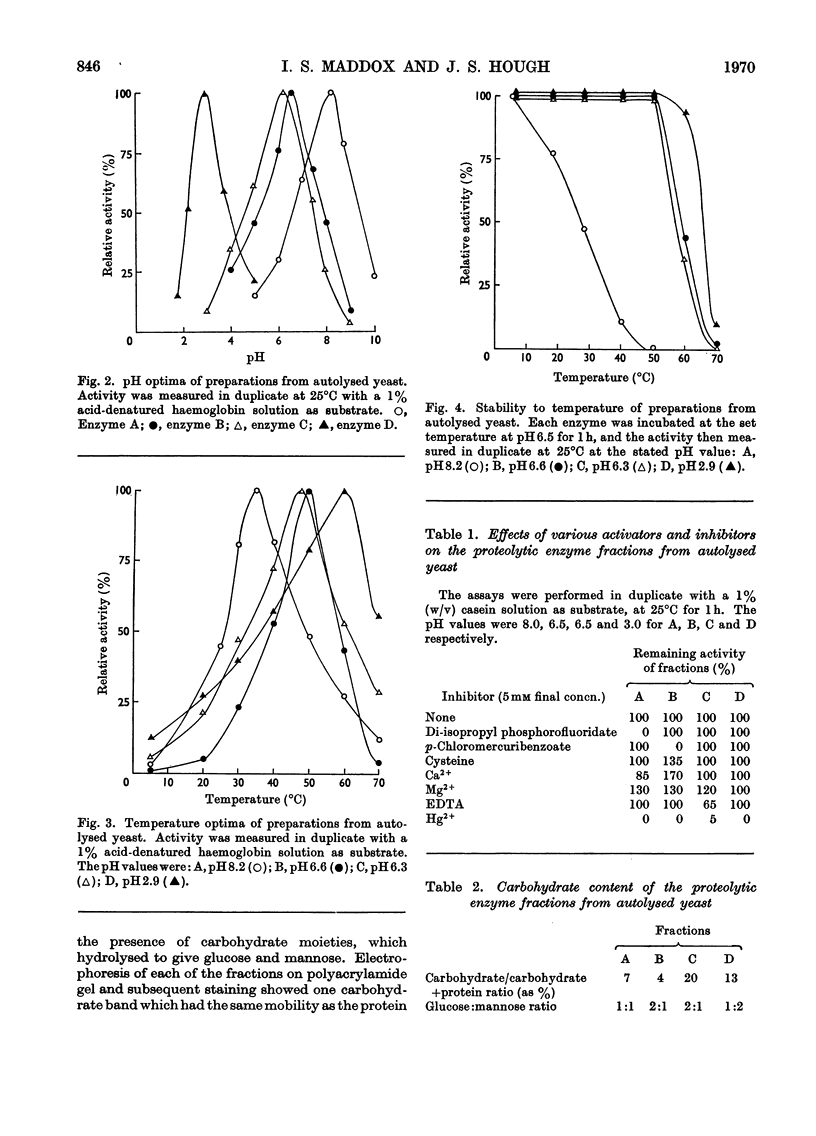
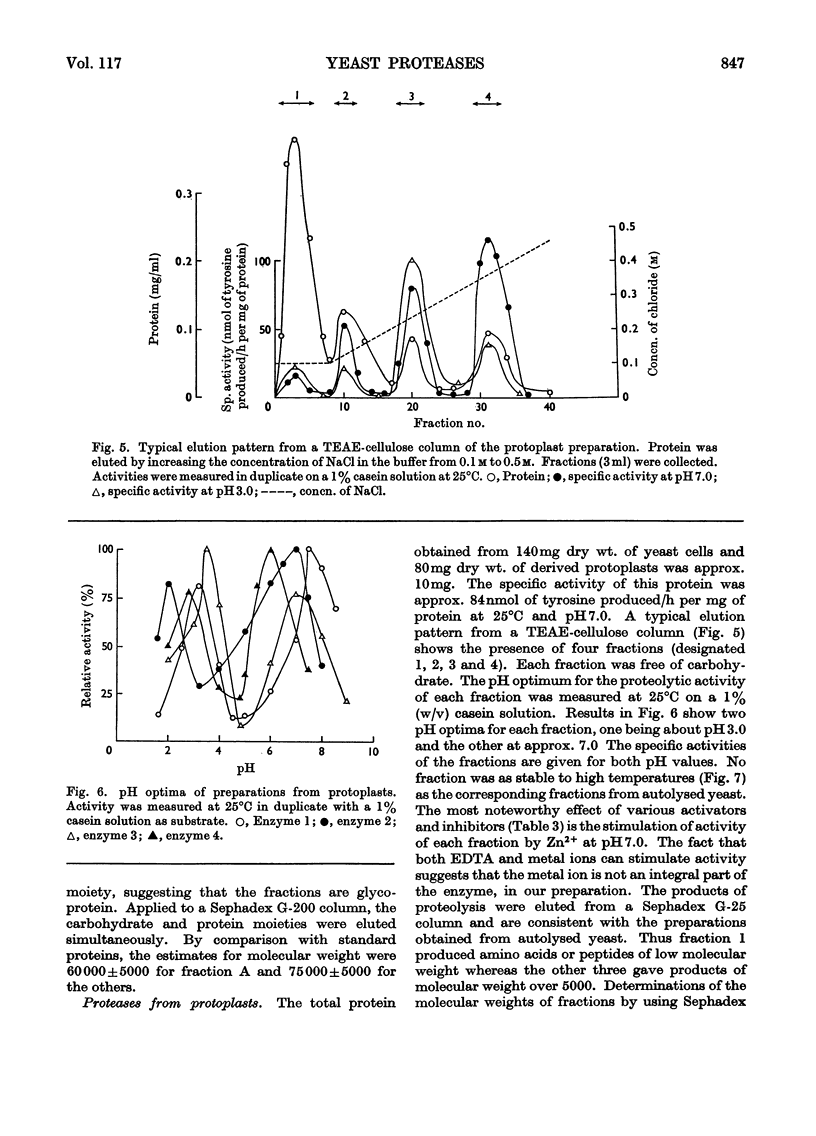
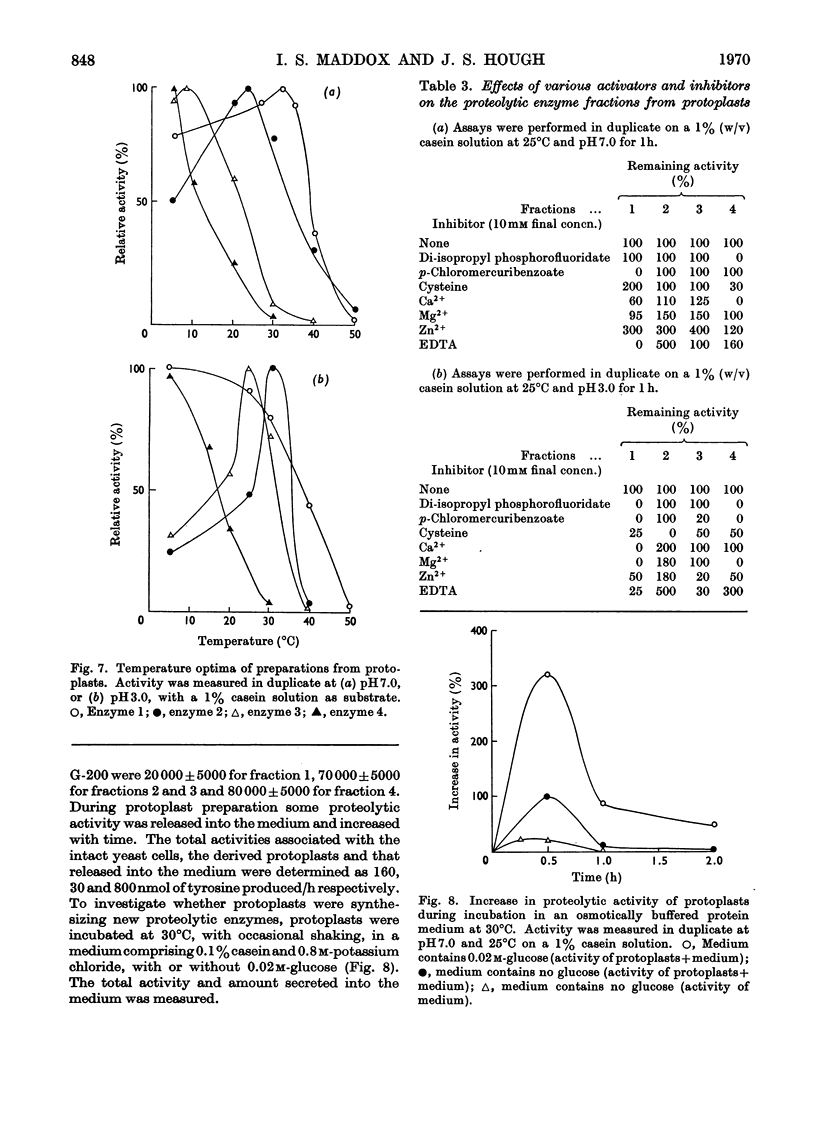
1. Of four proteolytic enzymes isolated from autolysing Saccharomyces carlsbergensis, one is inactivated at about 45°C, whereas the others are stable at 50°C. pH optima for activity are from 3.0 to 8.0 but maximum stability is between pH6.0 and 6.5. All appear to be glycoproteins, the carbohydrate moiety containing glucose and mannose residues. 2. Lysed protoplasts of the same yeast release four proteolytic enzymes each of which have two pH optima at pH3.0 and 7.0 approximately. Compared with the enzymes from autolysed yeast, resistance to high temperature is much less, and they are not glycoprotein in nature. 3. The same yeast grown with N-acetyltyrosine ethyl ester as nitrogen source secretes into the medium four proteases believed to be glycoprotein in nature. Generally they resemble the enzymes from lysed protoplasts more than those from autolysing yeast.
Full text
PDF

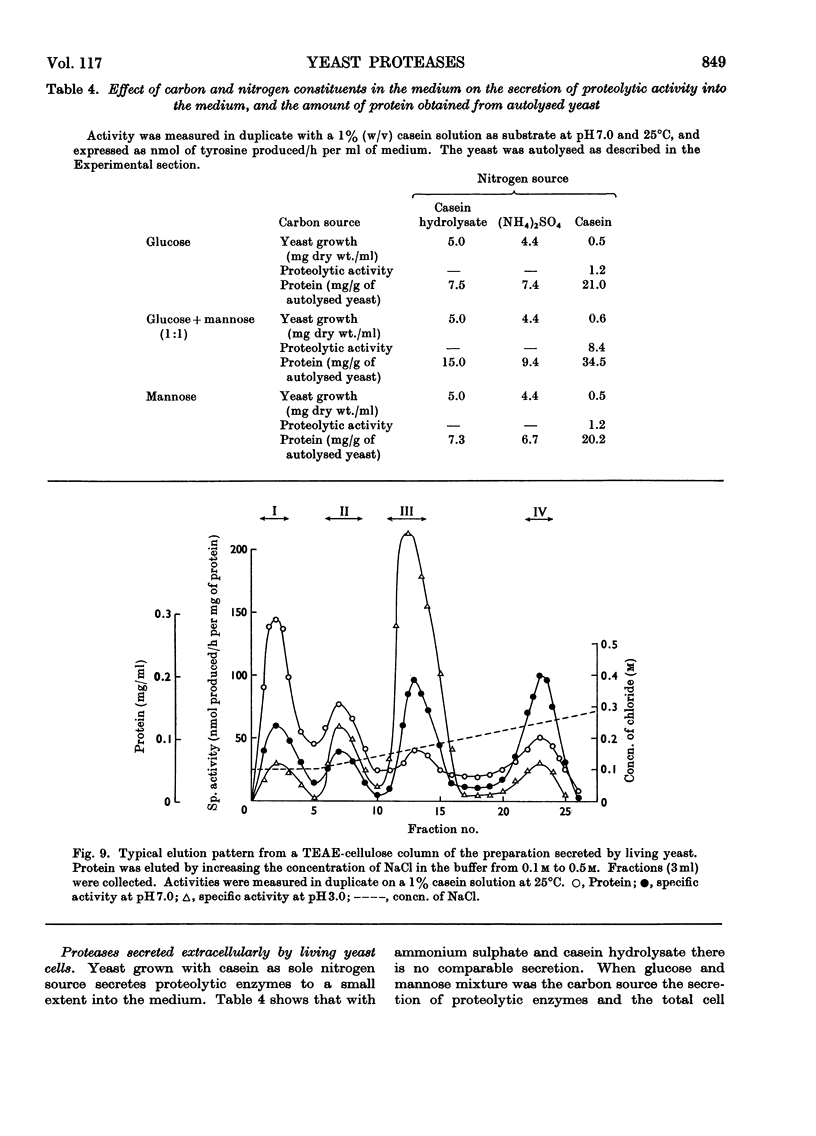
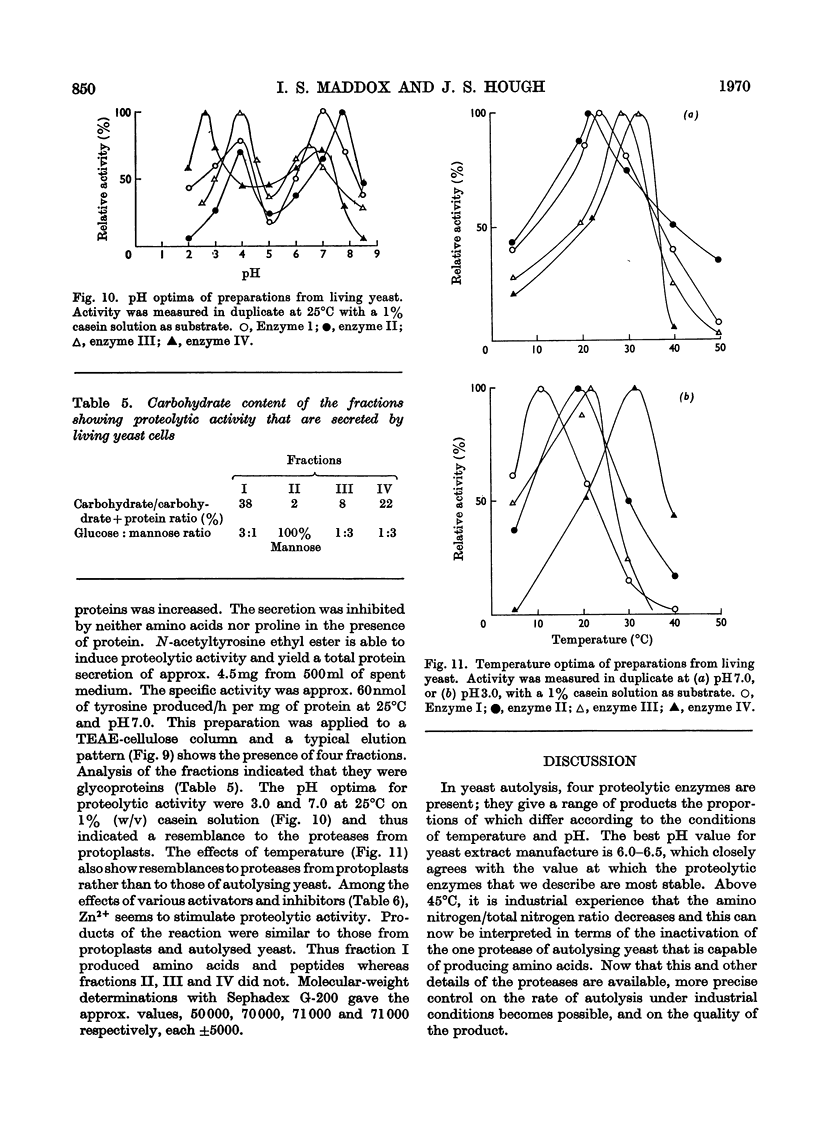


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akroyd P. Acrylamide gel slab electrophoresis in a simple glass cell for improved resolution and comparison of serum proteins. Anal Biochem. 1967 Jun;19(3):399–410. doi: 10.1016/0003-2697(67)90229-1. [DOI] [PubMed] [Google Scholar]
- CUTTS N. S., RAINBOW C. Studies of a yeast exacting towards p-aminobenzoic acid. J Gen Microbiol. 1950 May;4(2):150–155. doi: 10.1099/00221287-4-2-150. [DOI] [PubMed] [Google Scholar]
- Candy D. J. Occurrence and metabolism of scylloinositol in the locust. Biochem J. 1967 Jun;103(3):666–671. doi: 10.1042/bj1030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOSH A., CHARALAMPOUS F., SISON Y., BORER R. Metabolic function of myo-inositol. I. Cytological and chemical alterations in yeast resulting from inositol deficiency. J Biol Chem. 1960 Sep;235:2522–2528. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem J. 1967 Oct;105(1):189–203. doi: 10.1042/bj1050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARCIONE E. J., BOHNE M., LEAHY M. THE SUBECELLULAR SITE OF HEXOSAMINE INCORPORATION INTO LIVER PROTEIN. Biochemistry. 1964 Dec;3:1973–1976. doi: 10.1021/bi00900a032. [DOI] [PubMed] [Google Scholar]
- Schulze I. T., Colowick S. P. The modification of yeast hexokinases by proteases and its relationship to the dissociation of hexokinase into subunits. J Biol Chem. 1969 May 10;244(9):2306–2316. [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The formation of buds in yeast. J Gen Microbiol. 1969 Mar;55(3):393–398. doi: 10.1099/00221287-55-3-393. [DOI] [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968 Sep;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. M., Kern M. The synthesis and secretion of gamma-globulin by lymph node cells. 3. The slow acquisition of the carbohydrate moiety of gamma-globulin and its relationship to secretion. Proc Natl Acad Sci U S A. 1968 Feb;59(2):546–553. doi: 10.1073/pnas.59.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]


