Abstract
A model is presented that accounts for all types of reversible inhibition by a single inhibitor molecule in bimolecular rapid-equilibrium random-order enzyme systems. The characterization of inhibition mechanisms by graphical methods is examined, and a system of nomenclature is suggested.
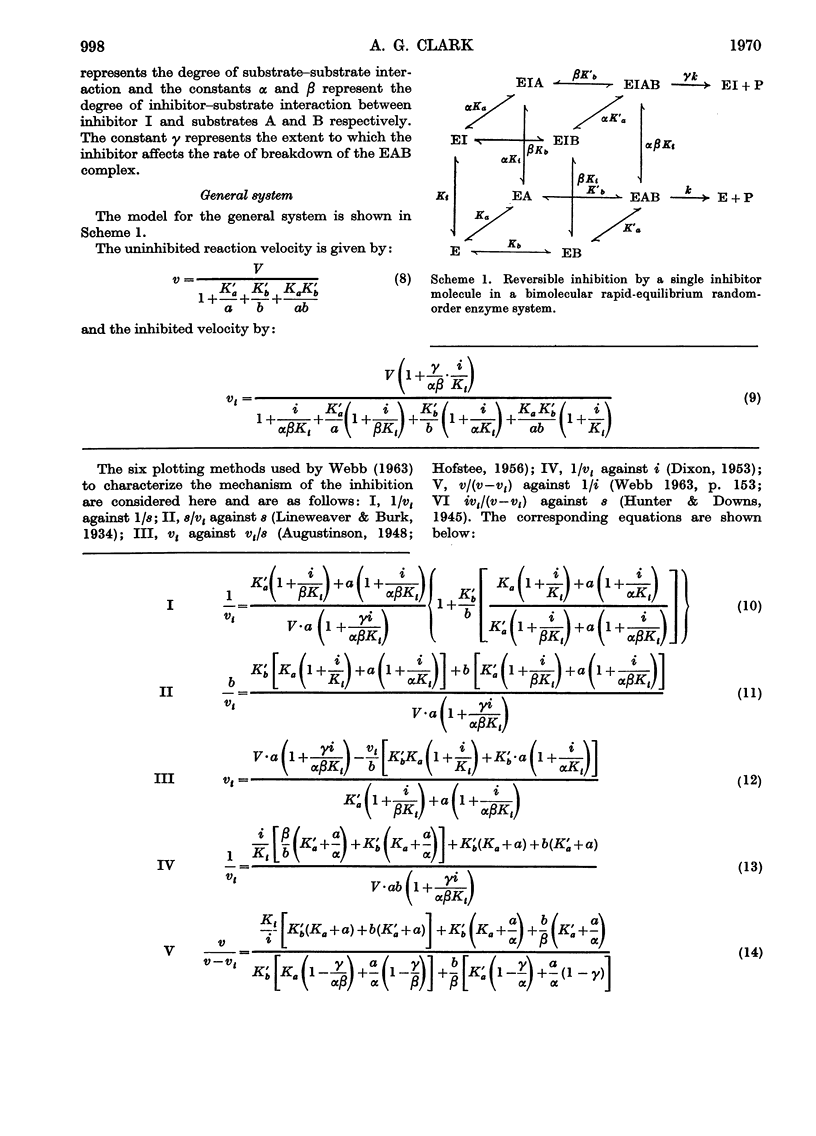
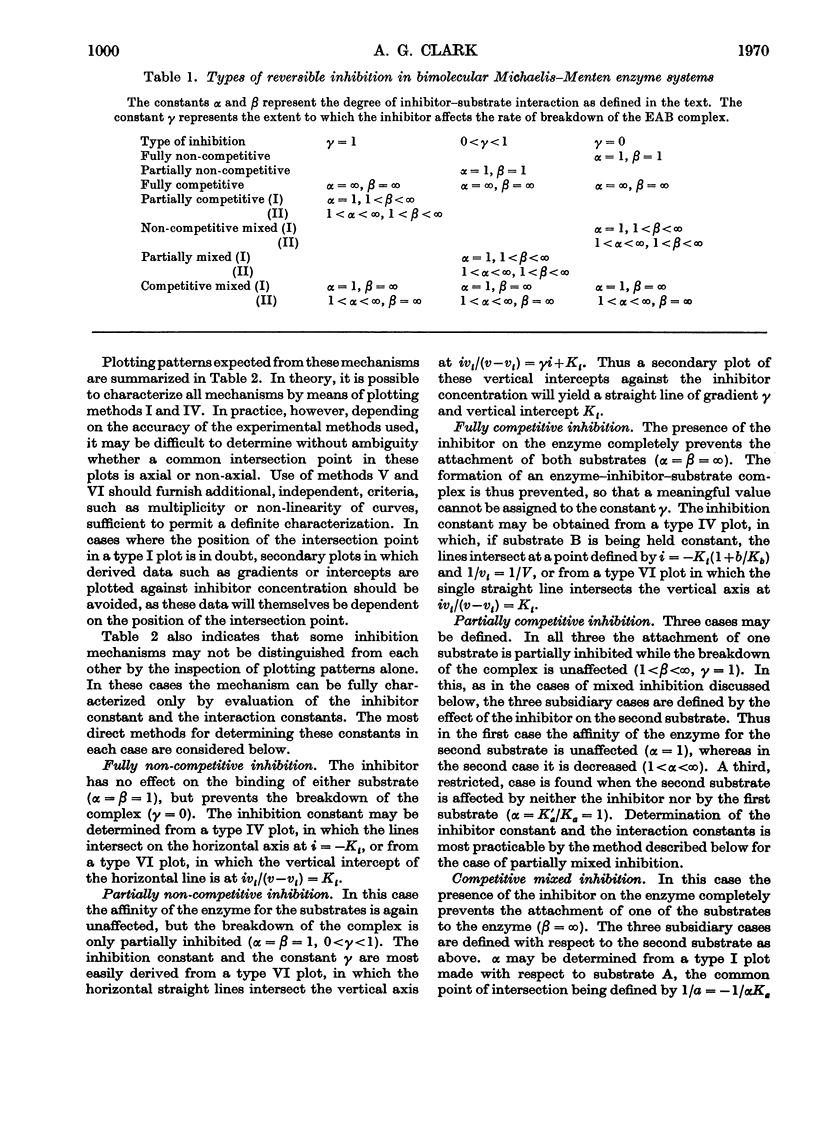
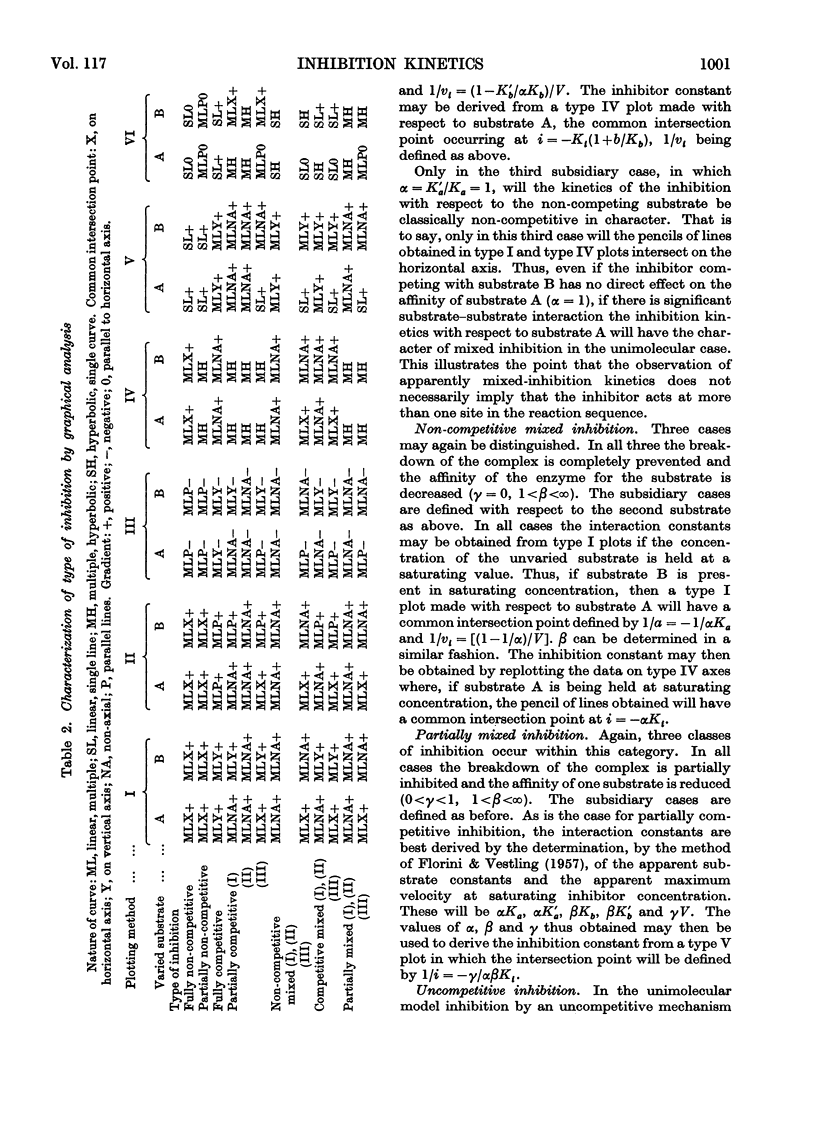
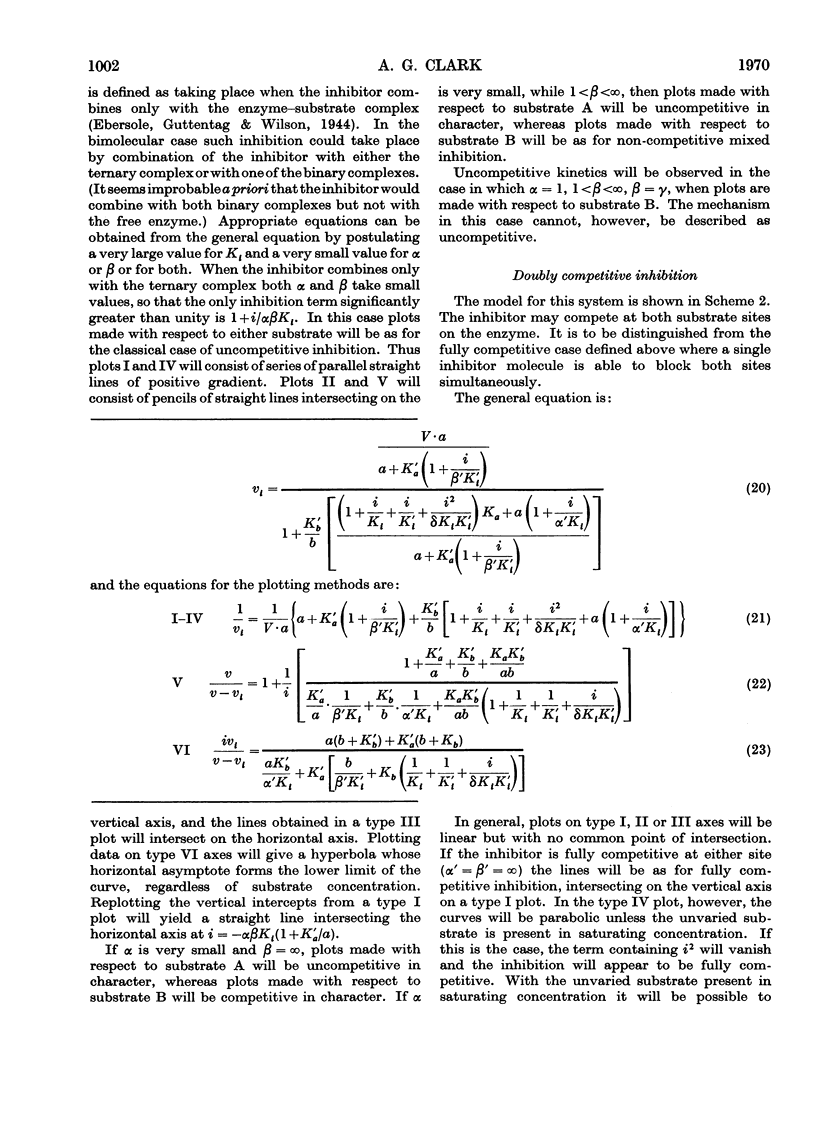
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balabaskaran S., Smith J. N. The inhibition of 1,1,1-trichloro-2,2-bis-(p-chlorophenyl)ethane (DDT) dehydrochlorinase and glutathione S-aryltransferase in grass-grub and housefly preparations. Biochem J. 1970 May;117(5):989–996. doi: 10.1042/bj1170989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. Enzymes catalysing conjugations of glutathione with alpha-beta-unsaturated carbonyl compounds. Biochem J. 1968 Oct;109(4):651–661. doi: 10.1042/bj1090651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Clark A. G., Darby F. J., Smith J. N. Species differences in the inhibition of glutathione S-aryltransferase by phthaleins and dicarboxylic acids. Biochem J. 1967 Apr;103(1):49–54. doi: 10.1042/bj1030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. J., Smith J. N., Turbert H. Comparative detoxication. 10. The enzymic conjugation of chloro compounds with glutathione in locusts and other insects. Biochem J. 1964 Mar;90(3):457–464. doi: 10.1042/bj0900457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. TREATMENT OF ENZYME KINETIC DATA. I. THE EFFECT OF MODIFIERS ON THE KINETIC PARAMETERS OF SINGLE SUBSTRATE ENZYMERS. J Biol Chem. 1964 Oct;239:3522–3531. [PubMed] [Google Scholar]
- HOFSTEE B. H. Graphical analysis of single enzyme systems. Enzymologia. 1956 Jul 15;17(5-6):273–278. [PubMed] [Google Scholar]


