Abstract
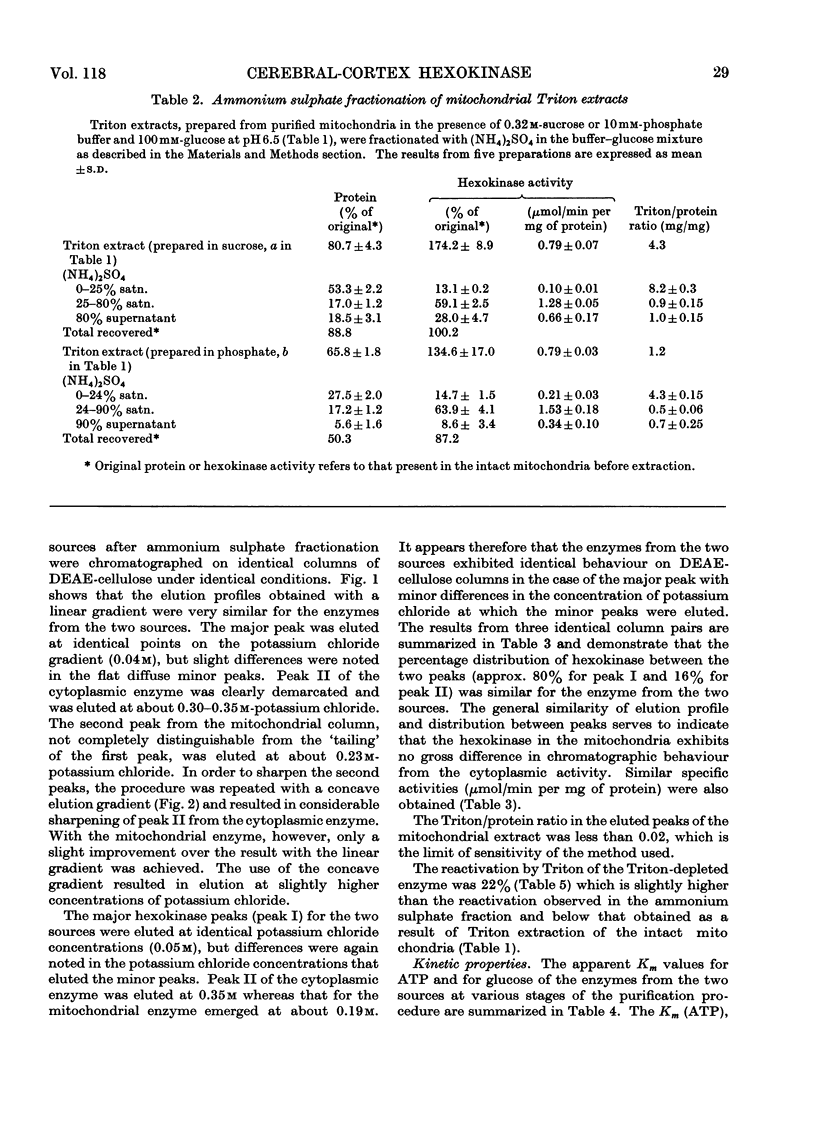
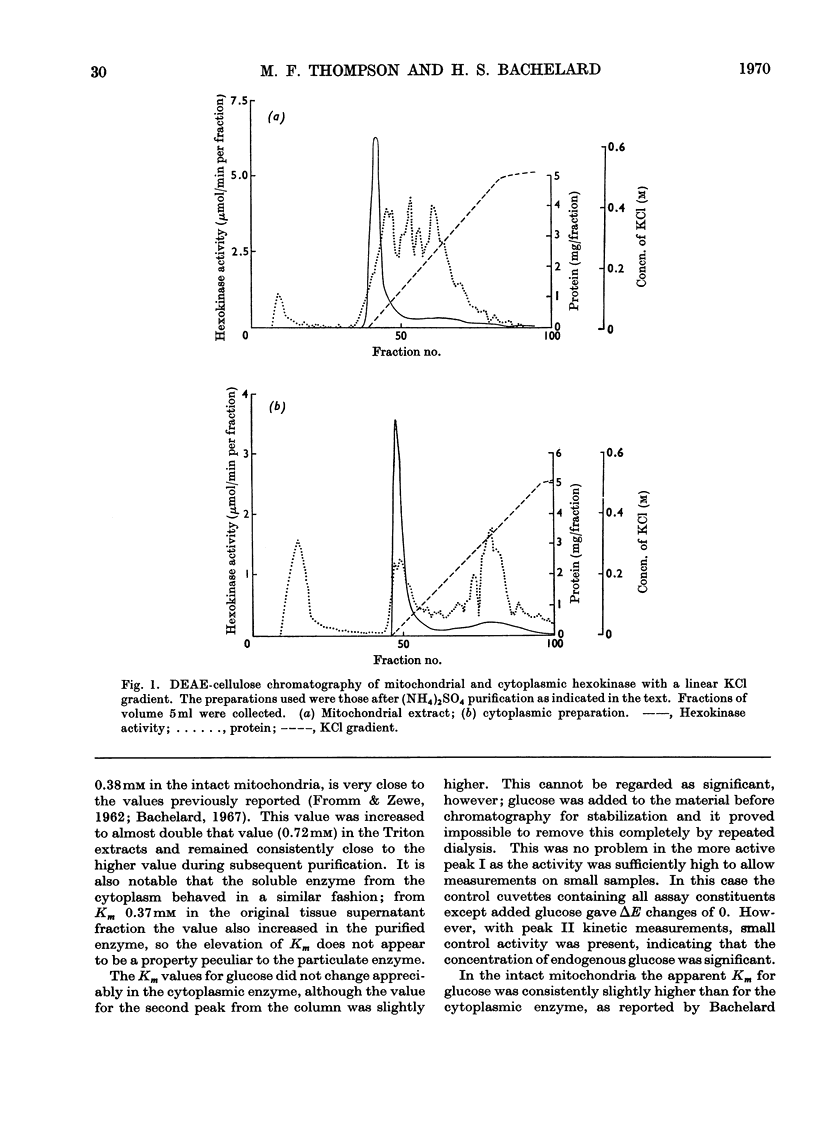
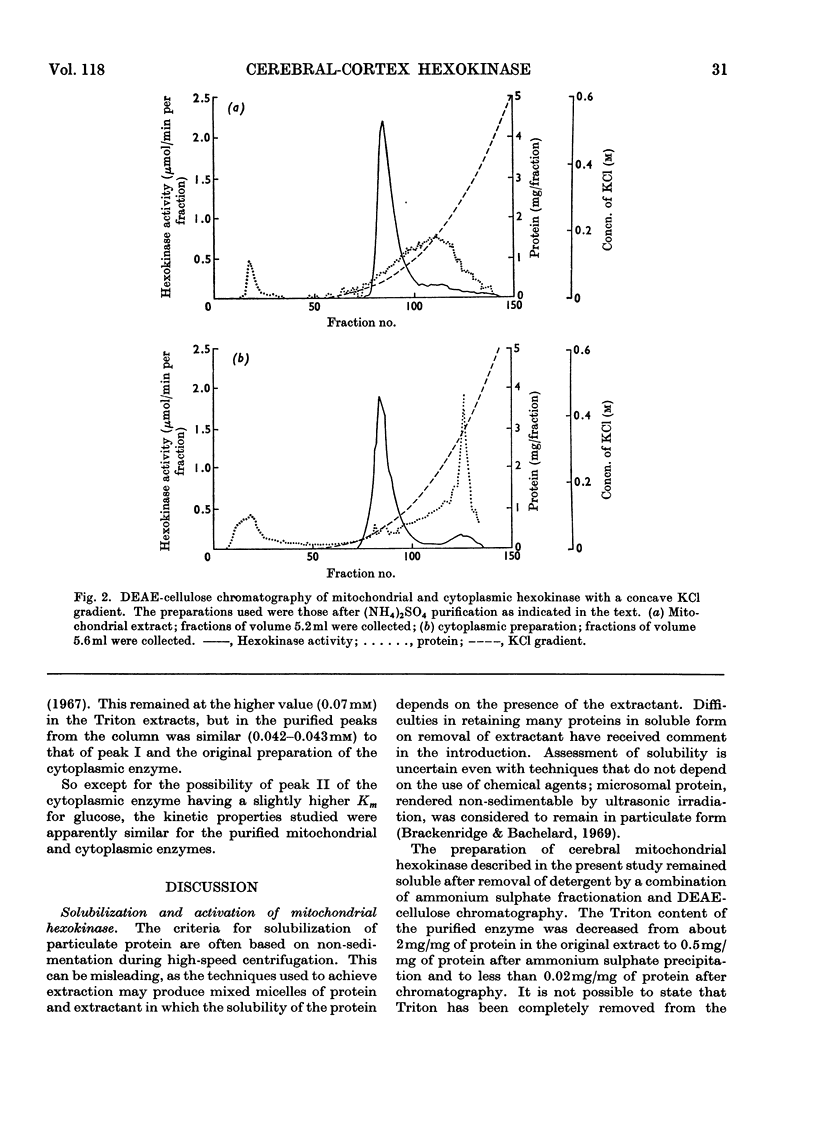
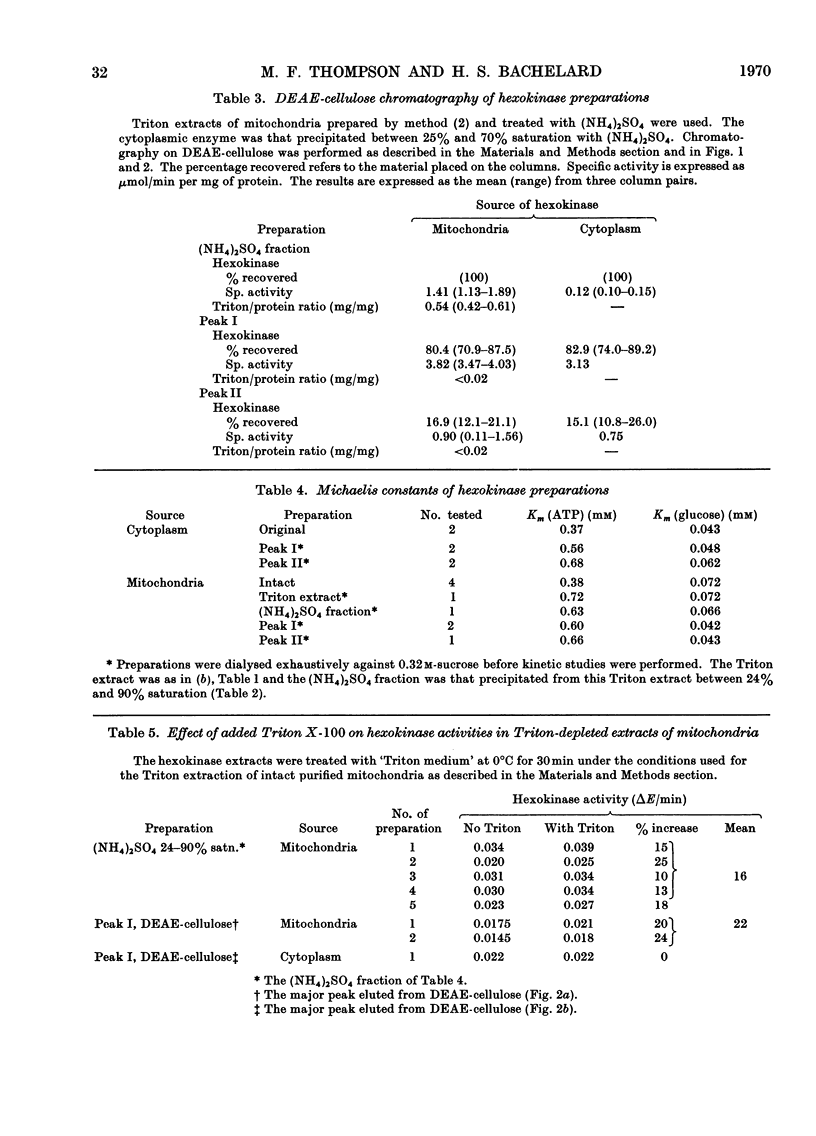
1. Cerebral-cortex mitochondria, after purification by using high-density sucrose solutions, were extracted with Triton X-100. The total hexokinase activity of the intact mitochondria was increased by 50–80% in the Triton extracts. 2. Triton X-100 was removed from mitochondrial extracts by a combination of ammonium sulphate fractionation and DEAE-cellulose chromatography. Mitochondrial hexokinase remained soluble after removal of extractant. 3. The behaviour of solubilized mitochondrial hexokinase was compared with soluble cytoplasmic hexokinase from the same samples of cerebral cortex on identical columns of DEAE-cellulose. Two peaks were eluted from each source of hexokinase. The distribution between hexokinase peaks was similar for the two sources. Peak I (approx. 80% of the total hexokinase) from each was eluted at identical concentrations of potassium chloride and slight differences were observed in the elution profiles for peak II. 4. The purified mitochondrial hexokinase showed the following kinetic properties: peak I, Km(ATP) 0.60mm, Km(glucose) 0.042mm; peak II, Km(ATP) 0.66mm, Km(glucose) 0.043mm. The purified cytoplasmic hexokinase Michaelis constants were: peak I, Km(ATP) 0.56mm, Km(glucose) 0.048mm; peak II, Km(ATP) 0.68mm, Km(glucose) 0.062mm. 5. Although no significant differences between mitochondrial and cytoplasmic hexokinases were noted in chromatographic behaviour or in the kinetic properties studied, the purified mitochondrial enzyme was activated slightly (approx. 20%) by Triton X-100, in contrast with the cytoplasmic enzyme, which was not affected. 6. The results, taken to indicate basic similarity between mitochondrial and cytoplasmic hexokinases, are discussed in relation to the role of the two sources of enzyme in the metabolism of the tissue.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachelard H. S., Goldfarb P. S. Adenine nucleotides and magnesium ions in relation to control of mammalian cerebral-cortex hexokinase. Biochem J. 1969 May;112(5):579–586. doi: 10.1042/bj1120579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard H. S. The subcellular distribution and properties of hexokinases in the guinea-pig cerebral cortex. Biochem J. 1967 Jul;104(1):286–292. doi: 10.1042/bj1040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenridge C. J., Bachelard H. S. The extraction and some properties of membrane-bound proteins from ox cerebral cortex microsomes. Int J Protein Res. 1969;1(3):157–168. doi: 10.1111/j.1399-3011.1969.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Copley M., Fromm H. J. Kinetic studies of the brain hexokinase reaction. A reinvestigation with the solubilized bovine enzyme. Biochemistry. 1967 Nov;6(11):3503–3509. doi: 10.1021/bi00863a023. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Thorne C. J. Preparation and some properties of L-3-glycerophosphate dehydrogenase from pig brain mitochondria. Biochem J. 1969 Jan;111(1):27–34. doi: 10.1042/bj1110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of the brain hexokinase reaction. J Biol Chem. 1962 May;237:1661–1667. [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- King L. J., Lowry O. H., Passonneau J. V., Venson V. Effects of convulsants on energy reserves in the cerebral cortex. J Neurochem. 1967 Jun;14(6):599–611. doi: 10.1111/j.1471-4159.1967.tb09563.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martonosi A. Sarcoplasmic reticulum. IV. Solubilization of microsomal adenosine triphosphatase. J Biol Chem. 1968 Jan 10;243(1):71–81. [PubMed] [Google Scholar]
- Newsholme E. A., Rolleston F. S., Taylor K. Factors affecting the glucose 6-phosphate inhibition of hexokinase from cerebral cortex tissue of the guinea pig. Biochem J. 1968 Jan;106(1):193–201. doi: 10.1042/bj1060193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas P. C., Bachelard H. S. The separation, prtial purificatio nd some properties of isoenzymes of aldolase from guinea-pig cerebral cortex. Biochem J. 1969 May;112(5):587–594. doi: 10.1042/bj1120587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnight R., Weller M., Goldfarb P. S. Large scale preparation of a crude membrane fraction from ox brain. J Neurochem. 1969 Dec;16(12):1591–1597. doi: 10.1111/j.1471-4159.1969.tb10357.x. [DOI] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Mitochondrial hexokinase. Release, rebinding, and location. J Biol Chem. 1967 Apr 10;242(7):1635–1645. [PubMed] [Google Scholar]
- Schwartz G. P., Basford R. E. The isolation and purification of solubilized hexokinase from bovine brain. Biochemistry. 1967 Apr;6(4):1070–1079. doi: 10.1021/bi00856a016. [DOI] [PubMed] [Google Scholar]
- Teichgräber P., Biesold D. Properties of membrane-bound hexokinase in rat brain. J Neurochem. 1968 Sep;15(9):979–989. doi: 10.1111/j.1471-4159.1968.tb11641.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J Biol Chem. 1968 Jul 10;243(13):3640–3647. [PubMed] [Google Scholar]


