Abstract
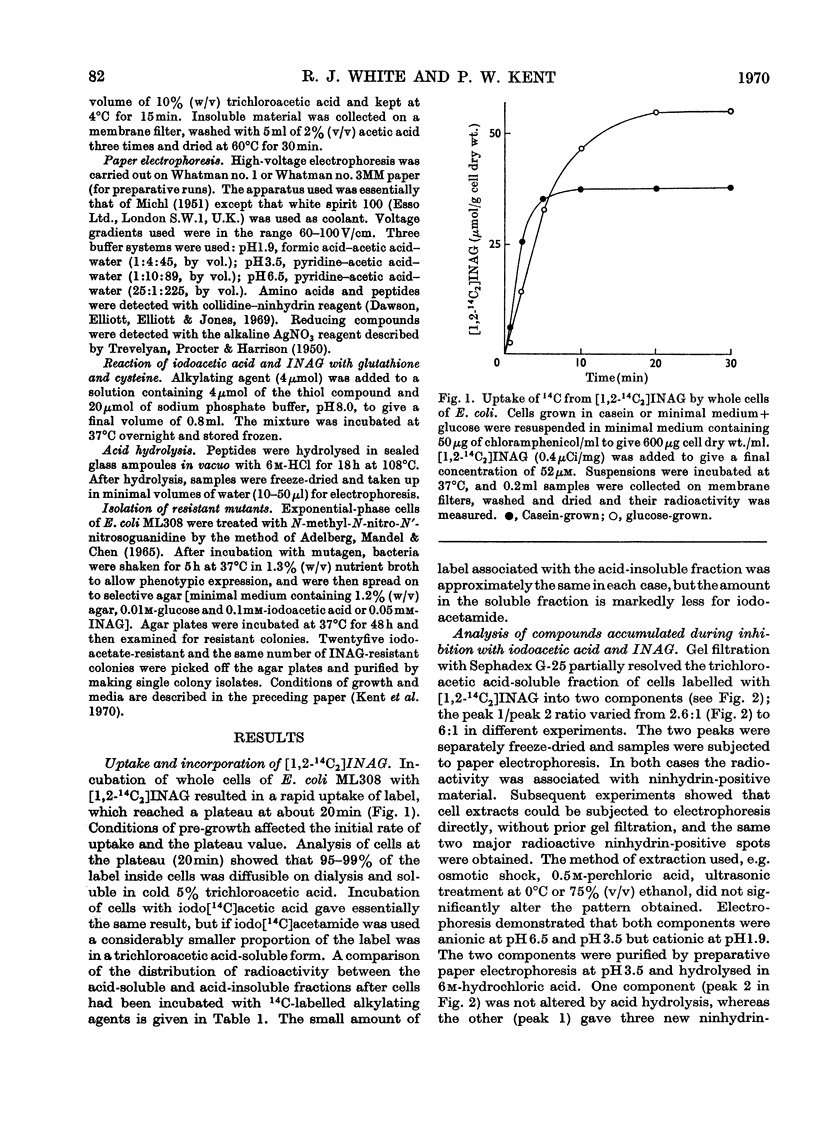
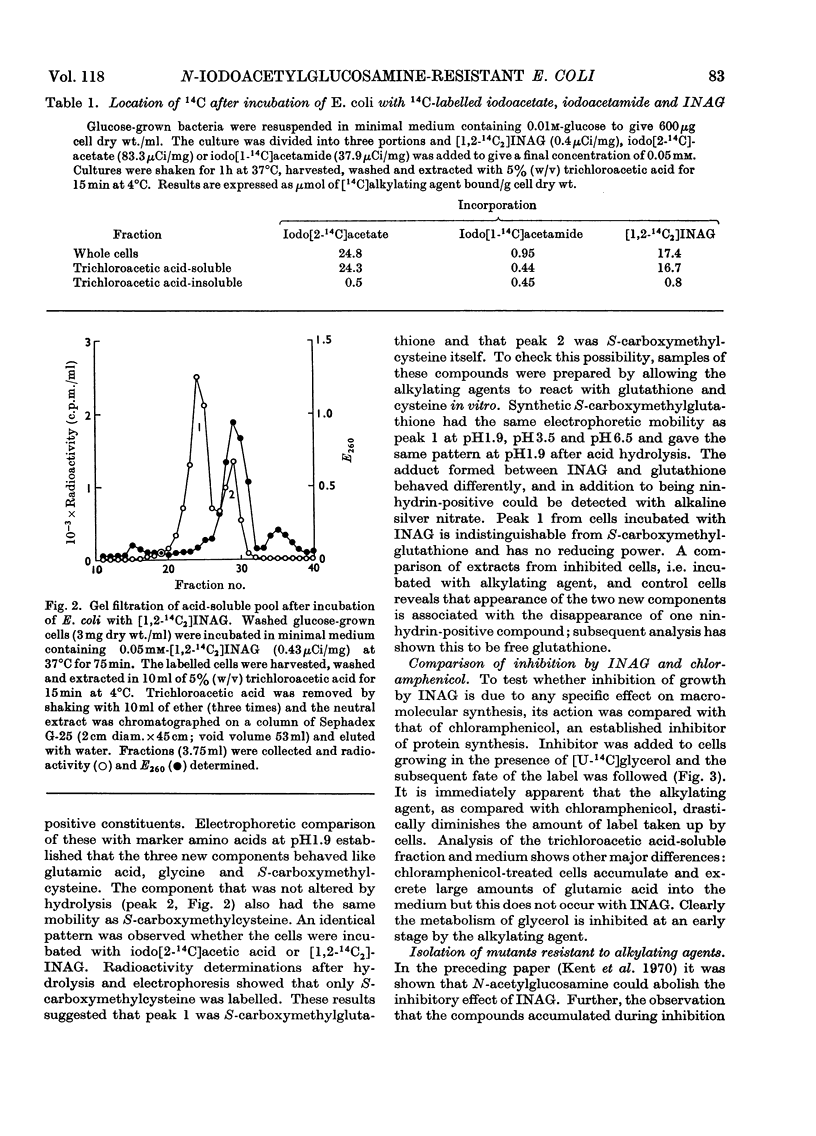
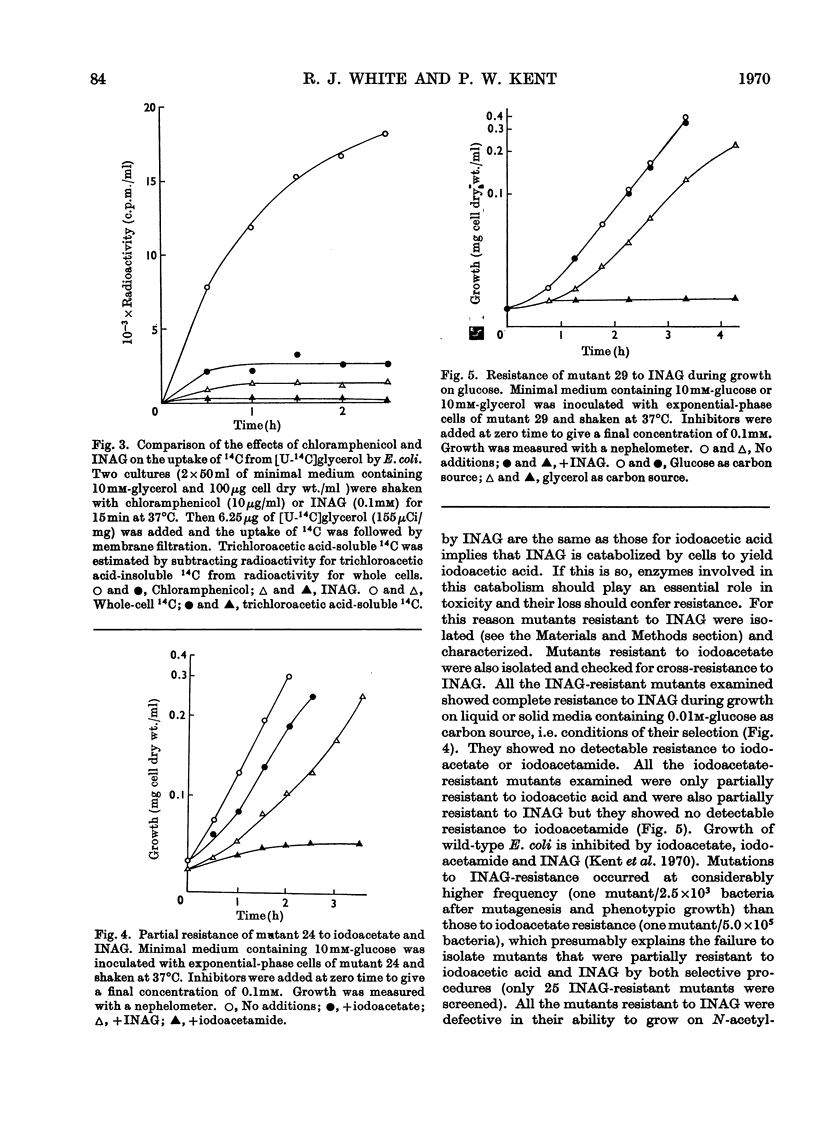
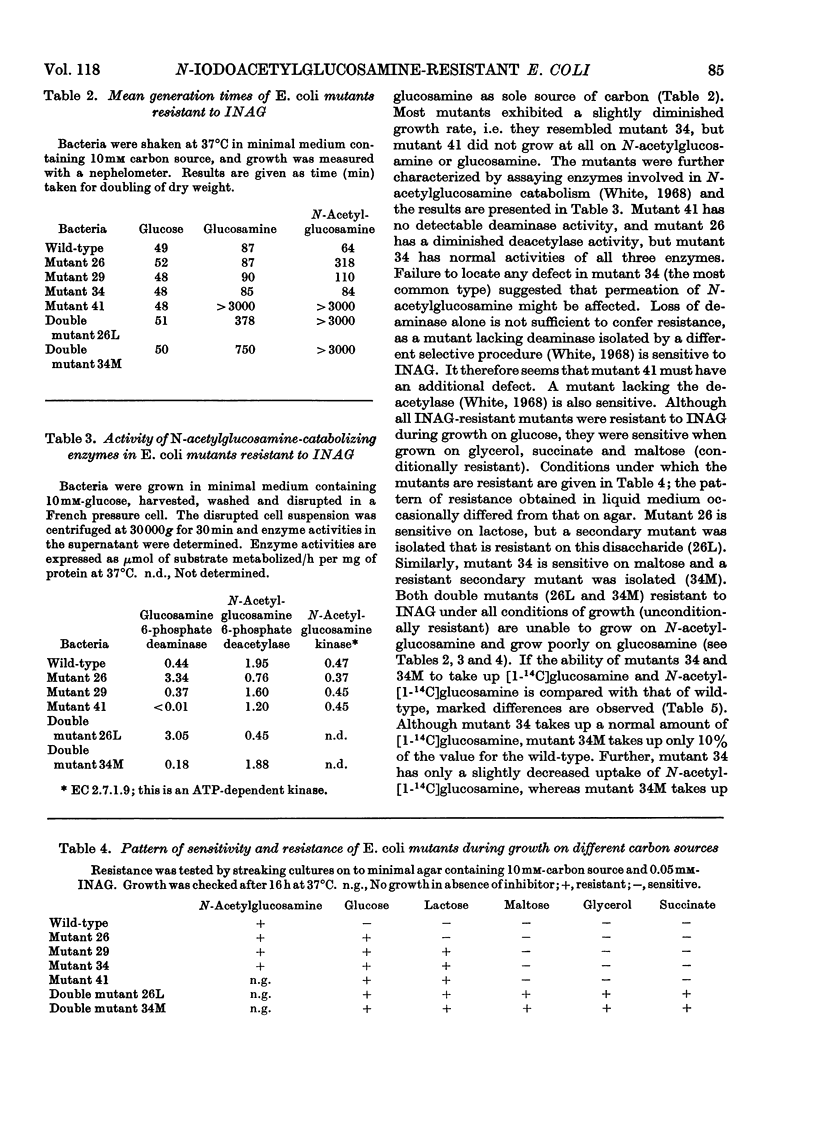
1. After incubation of Escherichia coli with N-iodo[1,2-14C2]acetylglucosamine, 95–99% of the 14C taken up by whole cells is located in a cold-trichloroacetic acid-soluble fraction. Two major components of this fraction are S-carboxymethylcysteine and S-carboxymethylglutathione. The same compounds accumulate during incubation with iodo[14C]acetate but not with iodo[14C]acetamide. The amount of 14C associated with a cold-trichloroacetic acid-insoluble fraction are comparable for all three alkylating agents. After incubation with iodo[14C]acetamide, 50% of the label bound to whole cells is recoverable in a cold-trichloroacetic acid-insoluble fraction. 2. Uptake and incorporation of 14C from [U-14C]glycerol is blocked at an early stage by N-iodoacetylglucosamine. No specific inhibition of macromolecular synthesis could be demonstrated. 3. Mutants selected for resistance to iodoacetate are partially resistant to iodoacetate and N-iodoacetylglucosamine, but show no resistance to iodoacetamide. 4. Mutants selected for resistance to N-iodoacetylglucosamine are not resistant to iodoacetate or iodoacetamide, and are defective in their ability to grow on N-acetylglucosamine. Resistance to N-iodoacetylglucosamine is not absolute, and depends on the presence of glucose or certain other sugars; there is no resistance during growth on maltose, glycerol or succinate. 5. Absolute resistance can be achieved by selecting for a second mutation conferring resistance during growth on maltose; double mutants isolated by this procedure are unable to grow on N-acetylglucosamine and grow poorly on glucosamine. Resistant single mutants have a slightly diminished uptake of N-acetyl[1-14C]glucosamine, but in resistant double mutants the uptake of both [1-14C]glucosamine and N-acetyl[1-14C]glucosamine is severely diminished. 6. These observations are consistent with the presence of two permeases for N-acetylglucosamine, one that also permits uptake of glucosamine and another that allows entry of methyl 2-acetamido-2-deoxy-α-d-glucoside. N-Iodoacetylglucosamine can gain entry to the cell by both permeases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F. Interaction of halogenacetates and SH compounds: The reaction of halogenacetic acids with glutathione and cysteine. The mechanism of iodoacetate poisoning of glyoxalase. Biochem J. 1933;27(4):1141–1151. doi: 10.1042/bj0271141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. I. General properties as studied in a galactokinaseless mutant. J Biol Chem. 1960 Jun;235:1580–1585. [PubMed] [Google Scholar]
- HUISMAN T. H., DOZY A. M. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med. 1962 Aug;60:302–319. [PubMed] [Google Scholar]
- Harding J. J. Glutathione-protein mixed disulphides in human lens. Biochem J. 1969 Oct;114(4):88P–89P. doi: 10.1042/bj1140088pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huishman T. H., Dozy A. M., Horton B. F., Nechtman C. M. Studies on the heterogeneity of hemoglobin. X. The nature of various minor hemoglobin components produced in human red blood cell hemolysates on aging. J Lab Clin Med. 1966 Mar;67(3):355–373. [PubMed] [Google Scholar]
- Jackson R. C., Harrap K. R., Smith C. A. Binding of glutathione and cysteine to cellular protein by mixed disulphide bonds. Biochem J. 1968 Dec;110(3):37P–37P. doi: 10.1042/bj1100037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent P. W., Ackers J. P., White R. J. N-iodoacetyl-D-glucosamine, an inhibitor of growth and glycoside uptake in Escherichia coli. Biochem J. 1970 Jun;118(1):73–79. doi: 10.1042/bj1180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Brookes P. Cytotoxicity of alkylating agents towards sensitive and resistant strains of Escherichia coli in relation to extent and mode of alkylation of cellular macromolecules and repair of alkylation lesions in deoxyribonucleic acids. Biochem J. 1968 Sep;109(3):433–447. doi: 10.1042/bj1090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZE C. E., THOMPSON T. E., LANGDON R. G. Hepatic glutathione reductase. II. Physical properties and mechanism of action. J Biol Chem. 1962 May;237:1596–1600. [PubMed] [Google Scholar]
- Modig H. Cellular mixed disulphides between thiols and proteins, and their possible implication for radiation protection. Biochem Pharmacol. 1968 Feb;17(2):177–186. doi: 10.1016/0006-2952(68)90321-3. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Kornberg H. L. Regulation of sugar utilization by Aspergillus nidulans. Biochim Biophys Acta. 1968 Jun 24;158(3):491–493. doi: 10.1016/0304-4165(68)90312-7. [DOI] [PubMed] [Google Scholar]
- Révész L., Modig H. Cysteamine-induced increase of cellular glutathione-level: a new hypothesis of the radioprotective mechanism. Nature. 1965 Jul 24;207(995):430–431. doi: 10.1038/207430a0. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Wheldrake J. F. Intracellular concentration of cysteine in Escherichia coli and its relation to repression of the sulphate-activating enzymes. Biochem J. 1967 Nov;105(2):697–699. doi: 10.1042/bj1050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. The role of the phosphoenolpyruvate phosphotransferase system in the transport of N-acetyl-D-glucosamine by Escherichia coli. Biochem J. 1970 Jun;118(1):89–92. doi: 10.1042/bj1180089. [DOI] [PMC free article] [PubMed] [Google Scholar]


