Abstract
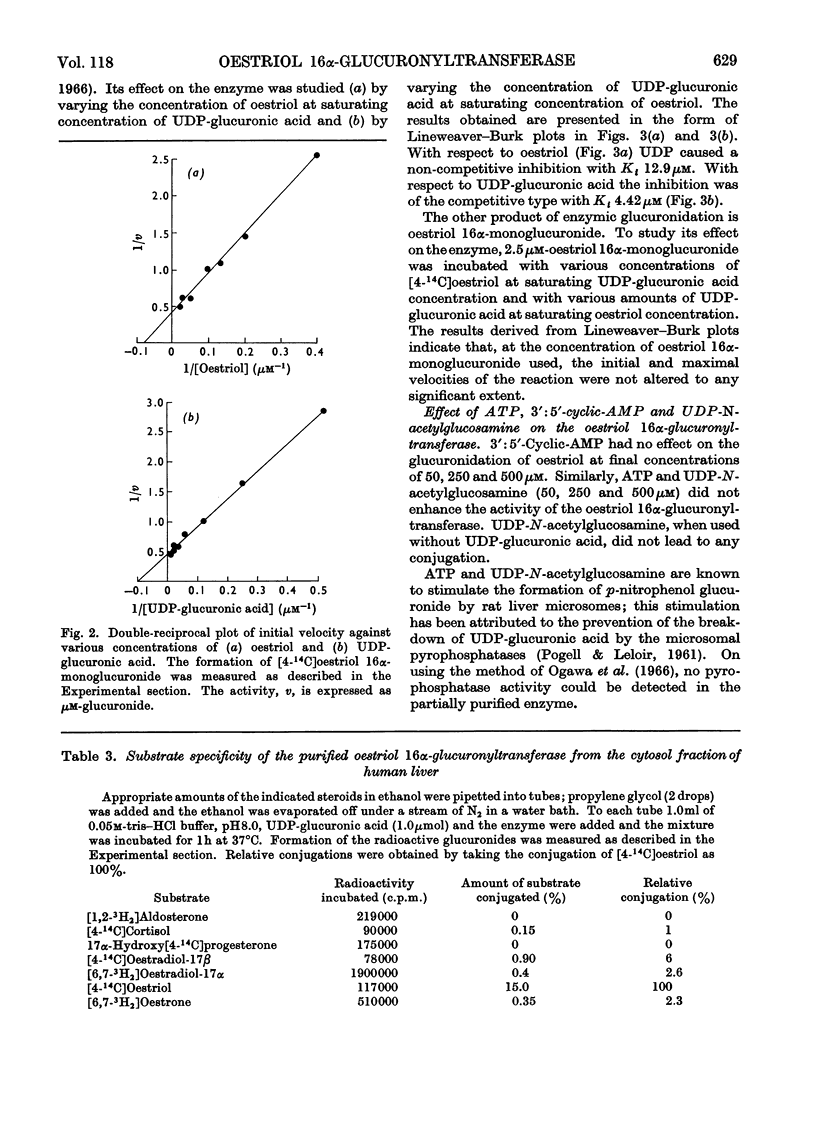
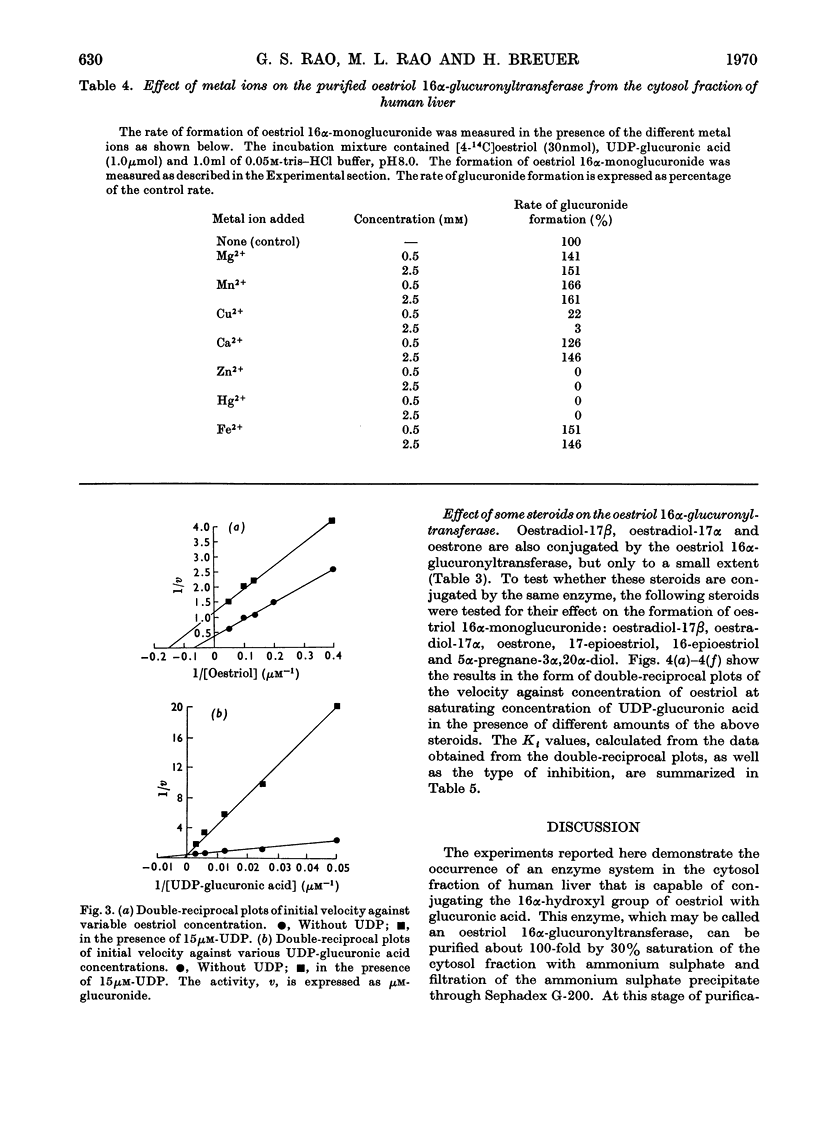
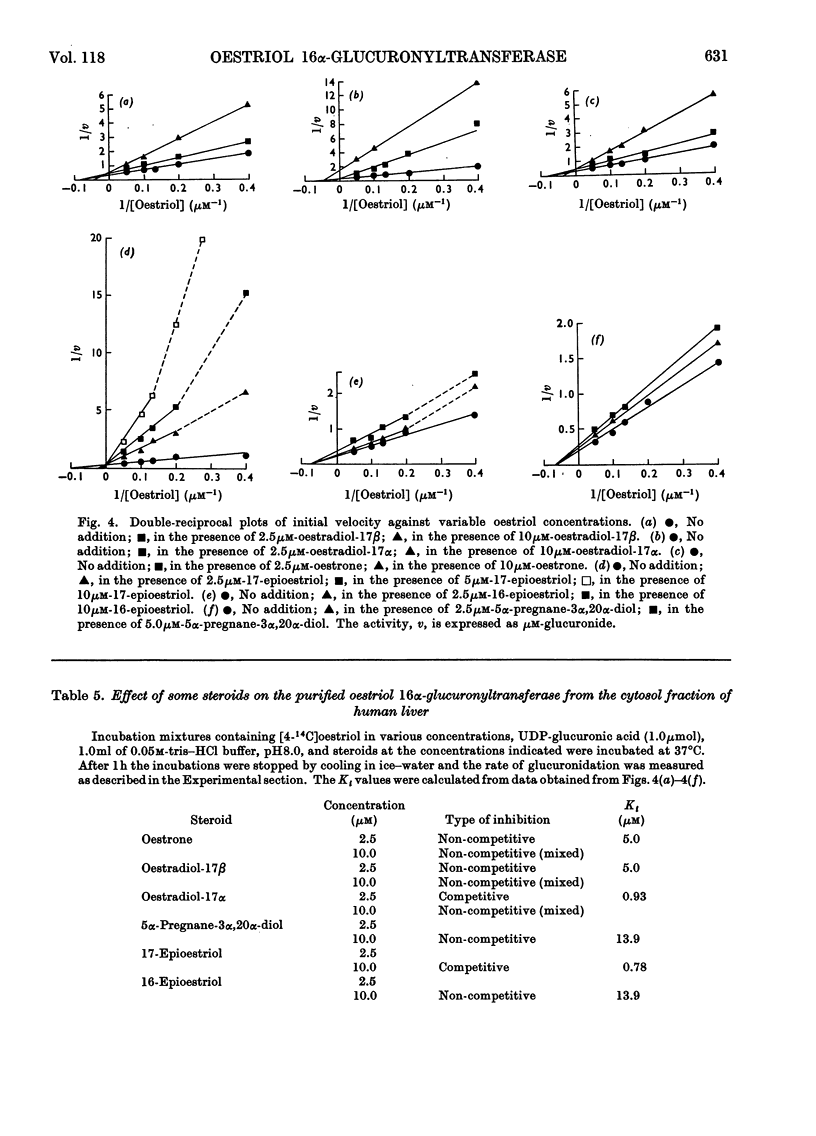
An enzyme that conjugates the 16α-hydroxyl group of oestriol with glucuronic acid was found in the cytosol fraction of human liver. The enzymic activity could not be sedimented when the cytosol fraction was centrifuged at 158000gav. for 120min. The oestriol 16α-glucuronyltransferase was purified 100-fold by 0–30% saturation of the cytosol fraction with ammonium sulphate followed by filtration of the precipitate through Sephadex G-200. The activity was eluted at the void volume. The product of the reaction, oestriol 16α-monoglucuronide, was identified by paper chromatography and by crystallization of radioactive product to constant specific radioactivity. The optimum temperature was 37°C, and the activation energy was calculated to be 11.1kcal/mol. The apparent Michaelis–Menten constants for oestriol and UDP-glucuronic acid were 13.3 and 100μm respectively. Cu2+, Zn2+ and Hg2+ inhibited, whereas Mg2+, Mn2+ and Fe2+ stimulated the enzyme. Substrate-specificity studies indicated that the amount of oestradiol-17β, oestradiol-17α and oestrone conjugated was not more than about 5% of that found for oestriol. Oestriol 16α-monoglucuronide, a product of the reaction, did not inhibit the 16α-oestriol glucuronyltransferase; in contrast, UDP, another product of the reaction, inhibited the enzyme competitively with respect to UDP-glucuronic acid as the substrate, and non-competitively with respect to oestriol as the substrate. ATP and UDP-N-acetylglucosamine did not affect the oestriol 16α-glucuronyltransferase. 17-Epioestriol acted as a competitive inhibitor and 16-epioestriol as a non-competitive inhibitor of the glucuronidation of oestriol. 5α-Pregnane-3α,20α-diol also inhibited the enzyme non-competitively. It is most likely that the oestriol 16α-glucuronyltransferase described here is bound to the membranes of the endoplasmic reticulum.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUTTON G. J., STOREY I. D. Uridine compounds in glucuronic acid metabolism. I. The formation of glucuronides in liver suspensions. Biochem J. 1954 Jun;57(2):275–283. doi: 10.1042/bj0570275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTTON G. J. Uridine diphosphate glucuronic acid as glucuronyl donor in the synthesis of ester, aliphatic and steroid glucuronides. Biochem J. 1956 Dec;64(4):693–701. doi: 10.1042/bj0640693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm K., Breuer H. Reinigung und Charakterisierung einer Löslichen Uridin Diphosphat-glucuronat:17-beta-Hydroxysteroid-Glucuronyl-Transferase beim Menschen. Biochim Biophys Acta. 1966 Nov 15;128(2):306–316. [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSELBACHER K. J., CHRABAS M. F., QUINN R. C. The solubilization and partial purification of a glucuronyl transferase from rabbit liver microsomes. J Biol Chem. 1962 Oct;237:3033–3036. [PubMed] [Google Scholar]
- ISSELBACHER K. J. Enzymatic mechanisms of hormone metabolism. II. Mechanism of hormonal glucuronide formation. Recent Prog Horm Res. 1956;12:134-46; discussion, 146-51. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leventer L. L., Buchanan J. L., Ross J. E., Tapley D. F. Solubilization of N-glucuronyl transferase. Biochim Biophys Acta. 1965 Nov 22;110(2):428–430. doi: 10.1016/s0926-6593(65)80052-2. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Spontaneous and detergent activation of a glucuronyltransferase in vitro. Arch Biochem Biophys. 1967 Apr;120(1):198–203. doi: 10.1016/0003-9861(67)90614-5. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Sawada M., Kawada M. Purification and properties of a pyrophosphatase from rat liver microsomes capable of catalyzing the hydrolysis of UDP-glucuronic acid. J Biochem. 1966 Feb;59(2):126–134. doi: 10.1093/oxfordjournals.jbchem.a128272. [DOI] [PubMed] [Google Scholar]
- POGELL B. M., LELOIR L. F. Nucleotide activation of liver microsomal glucuronidation. J Biol Chem. 1961 Feb;236:293–298. [PubMed] [Google Scholar]
- Rao G. S., Breuer H. Partial purification and kinetic properties of a soluble estrogen glucuronyltransferase from pig intestine. J Biol Chem. 1969 Oct 25;244(20):5521–5527. [PubMed] [Google Scholar]
- SLAUNWHITE W. R., Jr, LICHTMAN M. A., SANDBERG A. A. STUDIES ON PHENOLIC STEROIDS IN HUMAN SUBJECTS. VI. BIOSYNTHESIS OF ESTRIOLGLUCOSIDURONIC ACID-16-C-14 BY HUMAN LIVER. J Clin Endocrinol Metab. 1964 Jul;24:638–643. doi: 10.1210/jcem-24-7-638. [DOI] [PubMed] [Google Scholar]
- SMITH E. R., BREUER H. Enzymic formation of oestrone 3-glucuronide by rabbit-liver microsomes. Biochem J. 1963 Jul;88:168–175. doi: 10.1042/bj0880168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson G. A., Yaffe S. J. The formation of bilirubin and p-nitrophenyl glucuronides by rabbit liver. Biochem J. 1966 May;99(2):507–512. doi: 10.1042/bj0990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]


