Abstract
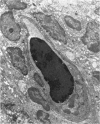
Kurloff cells of guinea pigs increase in number and accumulate in the spleen on oestrogen treatment. Because they contain metachromatic inclusions and are considered to be lymphocytes they were examined as a possible model for mucopolysaccharidoses like Hurler's syndrome, where some lymphocytes are also metachromatic. Oestrogen treatment produced a large increase in a glycosaminoglycan resembling chondroitin 4-sulphate in chemical analysis, chromatographic behaviour and i.r. spectrum but with an additional strong band at 805cm−1. Material isolated without proteolysis behaved on gel chromatography as a multiple-chain protein–polysaccharide whose molecular size was decreased by proteolysis. It contained xylose and galactose in molar proportions with serine, compatible with the presence of the same linkage region as in cartilage chondroitin 4-sulphate proteins and which likewise underwent alkaline β-elimination. Kurloff glycosaminoglycan chains were significantly longer than chondroitin sulphate chains of cartilage protein–polysaccharides as assessed by gel chromatography and the molar ratios of galactosamine to xylose or to serine. Kurloff cells thus contain intact rather than partially degraded protein–polysaccharide and hence are not analogous to Hurler cells, and their electron micrographs were also different. The purified Kurloff protein–polysaccharide and glycosaminoglycan isolated here has been shown by Marshall, Swettenham, Vernon-Roberts & Revell (1970) to be toxic specifically to macrophages at extremely low concentrations in vitro, unlike chondroitin sulphate of protein–polysaccharides from cartilage. The toxic constituent may account for the i.r.-absorption band at 805cm−1. Although active incorporation of [35S]sulphate occurs at early stages of Kurloff-cell induction (Marshall et al. 1970), the fully developed Kurloff cell studied here showed very low incorporation in vitro and in vivo, suggesting that the inclusions are specialized for the storage of the toxic material.
Full text
PDF


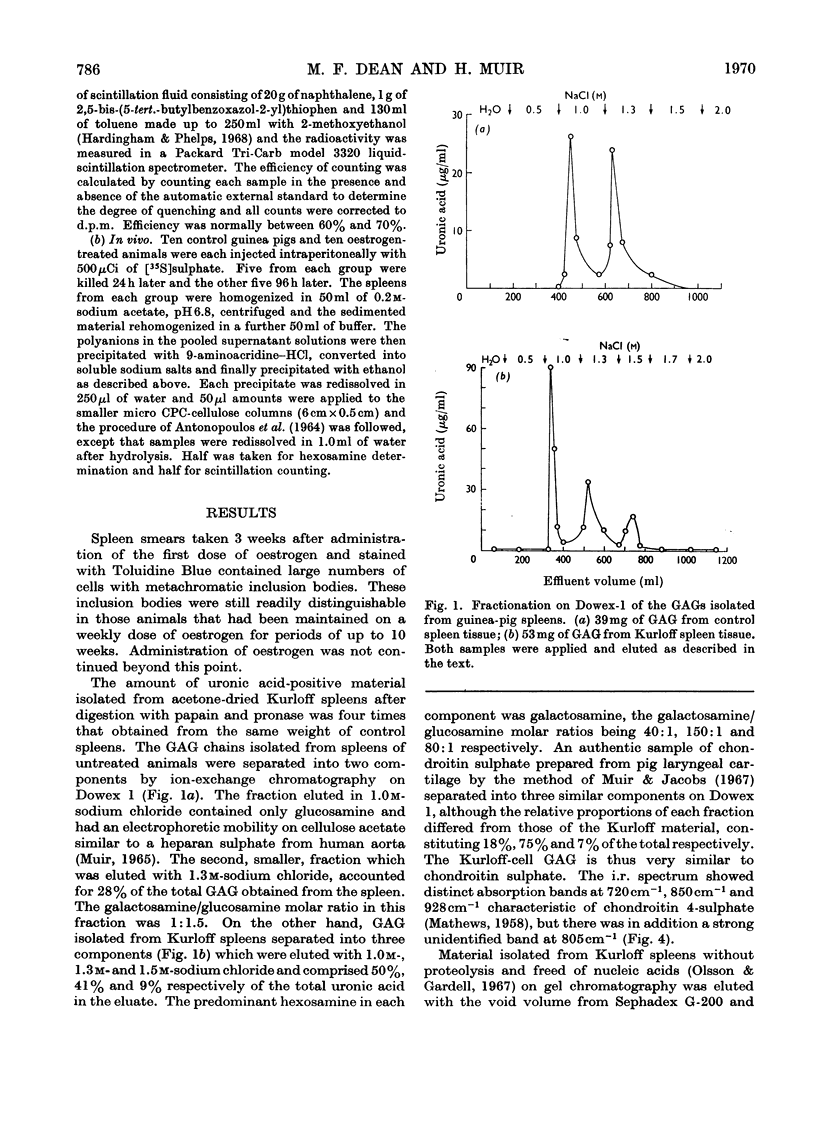
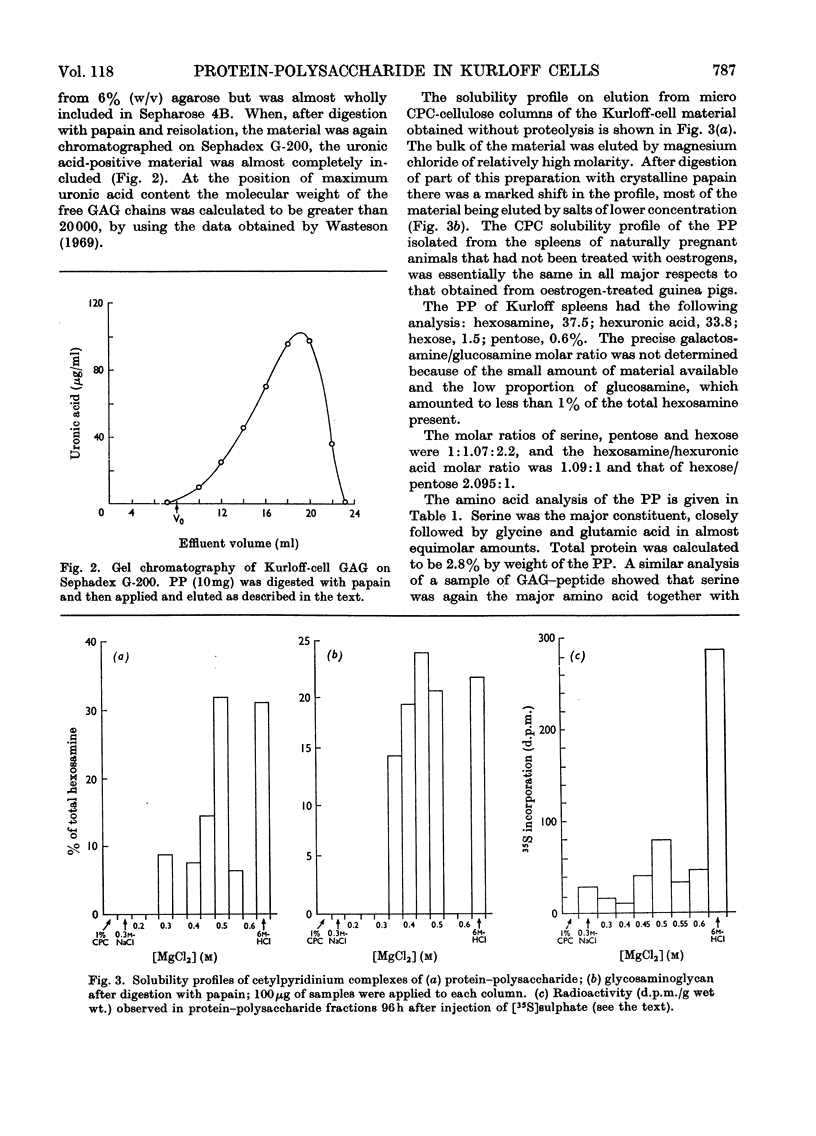

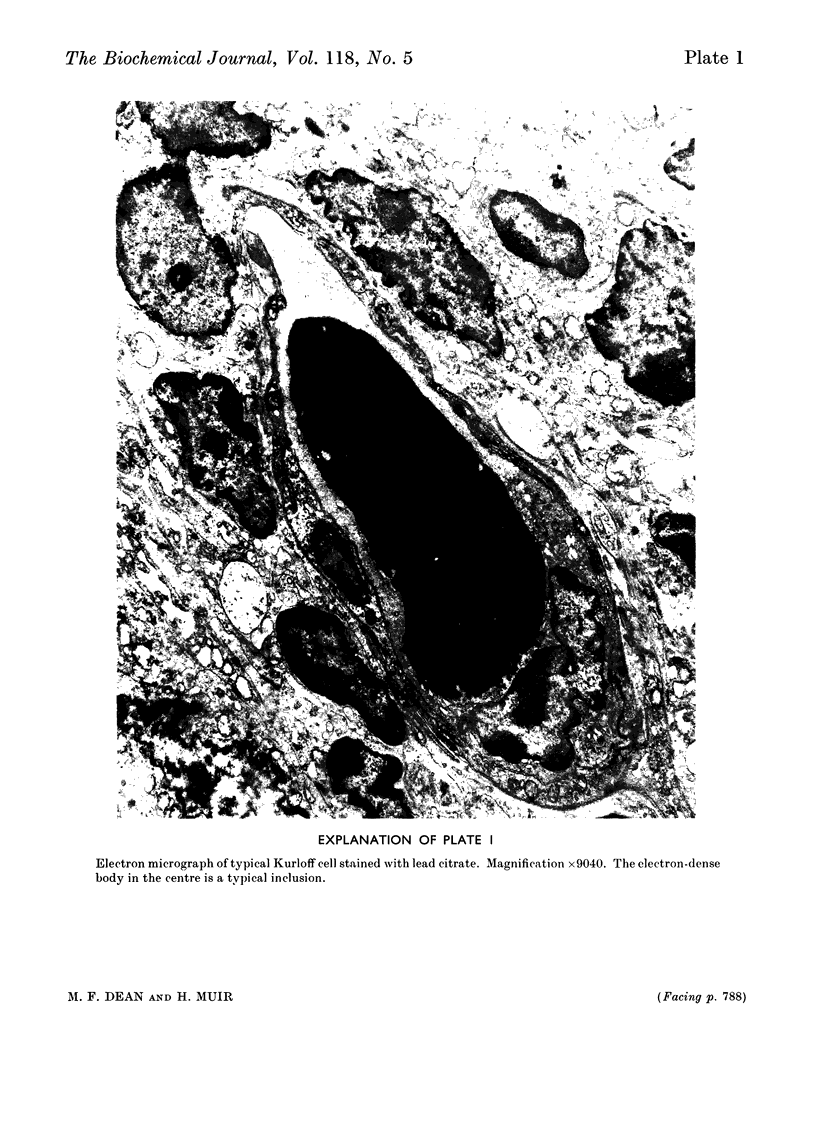
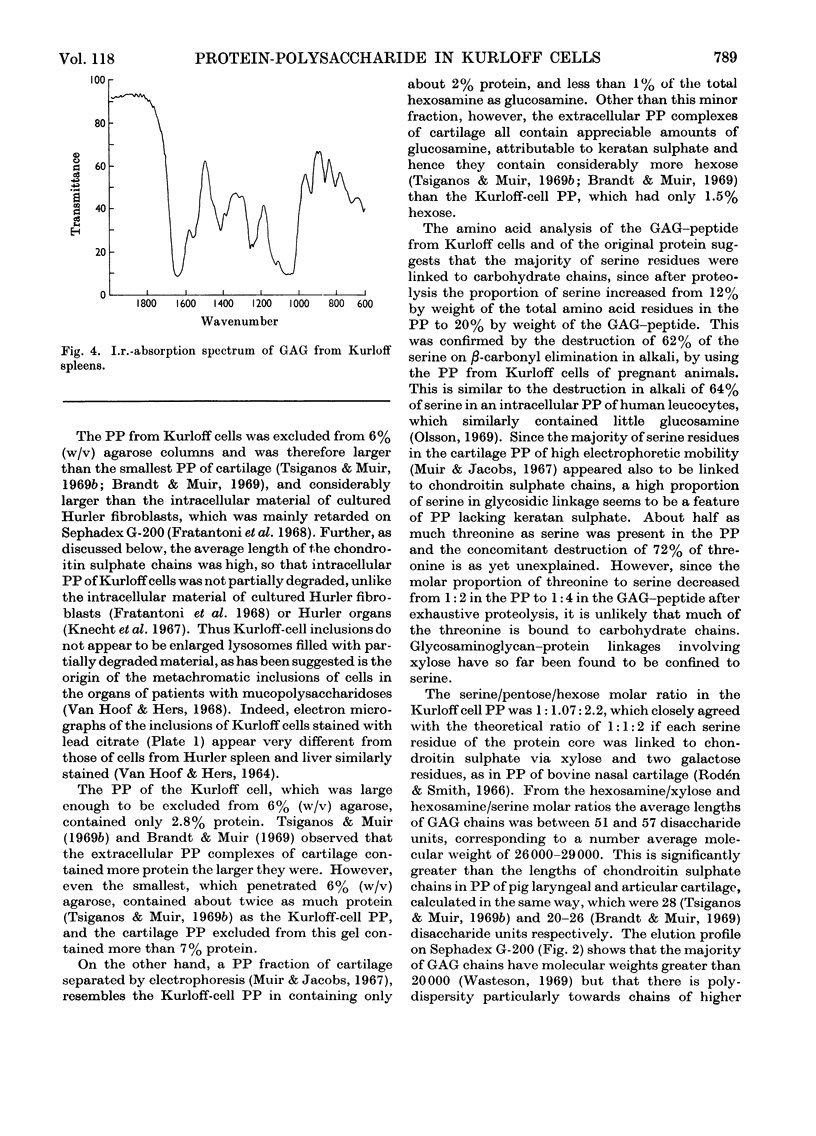

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONOPOULOS C. A., GARDELL S., SZIRMAI J. A., DETYSSONSK E. R. DETERMINATION OF GLYCOSAMINOGLYCANS (MUCOPOLYSACCHARIDES) FROM TISSUE ON THE MICROGRAM SCALE. Biochim Biophys Acta. 1964 Mar 2;83:1–19. doi: 10.1016/0926-6526(64)90045-x. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bitter T., Muir H. Mucopolysaccharides of whole human spleens in generalized amyloidosis. J Clin Invest. 1966 Jun;45(6):963–975. doi: 10.1172/JCI105412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Characterization of protein-polysaccharides of articular cartilage from mature and immature pigs. Biochem J. 1969 Oct;114(4):871–876. doi: 10.1042/bj1140871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M. F., Muir H. A chondroitin sulphate-protein in kurloff cells from guinea pig spleens. FEBS Lett. 1969 Aug;4(4):343–346. doi: 10.1016/0014-5793(69)80272-3. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Phelps C. F. The tissue content and turnover rates of intermediates in the biosynthesis of glycosaminoglycans in young rat skin. Biochem J. 1968 Jun;108(1):9–16. doi: 10.1042/bj1080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht J., Cifonelli J. A., Dorfman A. Structural studies on heparitin sulfate of normal and Hurler tissues. J Biol Chem. 1967 Oct 25;242(20):4652–4661. [PubMed] [Google Scholar]
- MARSHALL A. H., SWETTENHAM K. V. The formation of a mucoprotein-sulphated mucopolysaccharide complex in the lymphoid tissue of the pregnant guinea-pig. J Anat. 1959 Jul;93:348–353. [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. B. Isomeric chondroitin sulphates. Nature. 1958 Feb 8;181(4606):421–422. doi: 10.1038/181421a0. [DOI] [PubMed] [Google Scholar]
- MUIR H., MARSHALL A. H. Chemistry of a mucopolysaccharide produced by guinea pig lymphocytes. Nature. 1961 Aug 12;191:706–706. doi: 10.1038/191706a0. [DOI] [PubMed] [Google Scholar]
- MUIR H., MITTWOCH U., BITTER T. THE DIAGNOSTIC VALUE OF ISOLATED URINARY MUCOPOLYSACCHARIDES AND OF LYMPHOCYTE INCLUSIONS IN GARGOYLISM. Arch Dis Child. 1963 Aug;38:358–363. doi: 10.1136/adc.38.200.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H., Jacobs S. Protein-polysaccharides of pig laryngeal cartilage. Biochem J. 1967 May;103(2):367–374. doi: 10.1042/bj1030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I. Chondroitin sulfate proteolycan of human leukocytes. Biochim Biophys Acta. 1969 Apr 1;177(2):241–249. doi: 10.1016/0304-4165(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Olsson I., Gardell S. Isolation and characterization of glycosaminoglycans from human leukocytes and platelets. Biochim Biophys Acta. 1967 Jul 25;141(2):348–357. doi: 10.1016/0304-4165(67)90109-2. [DOI] [PubMed] [Google Scholar]
- Rodén L., Armand G. Structure of the chondroitin 4-sulfate-protein linkage region. Isolation and characterization of the disaccharide 3-O-beta-D-glucuronosyl-D-galactose. J Biol Chem. 1966 Jan 10;241(1):65–70. [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Estimation of pentoses and methylpentoses in biopolymers, in particular of fucose and xylose. Anal Biochem. 1966 Dec;17(3):495–501. doi: 10.1016/0003-2697(66)90184-9. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Extraction and purification. Biochem J. 1969 Aug;113(5):879–884. doi: 10.1042/bj1130879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof F., Hers H. G. The abnormalities of lysosomal enzymes in mucopolysacc- haridoses. Eur J Biochem. 1968 Dec;7(1):34–44. doi: 10.1111/j.1432-1033.1968.tb19570.x. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of molecular weight dispersion in chondroitin sulphate on a microgram level. Biochim Biophys Acta. 1969 Feb 18;177(1):152–154. doi: 10.1016/0304-4165(69)90076-2. [DOI] [PubMed] [Google Scholar]