Abstract
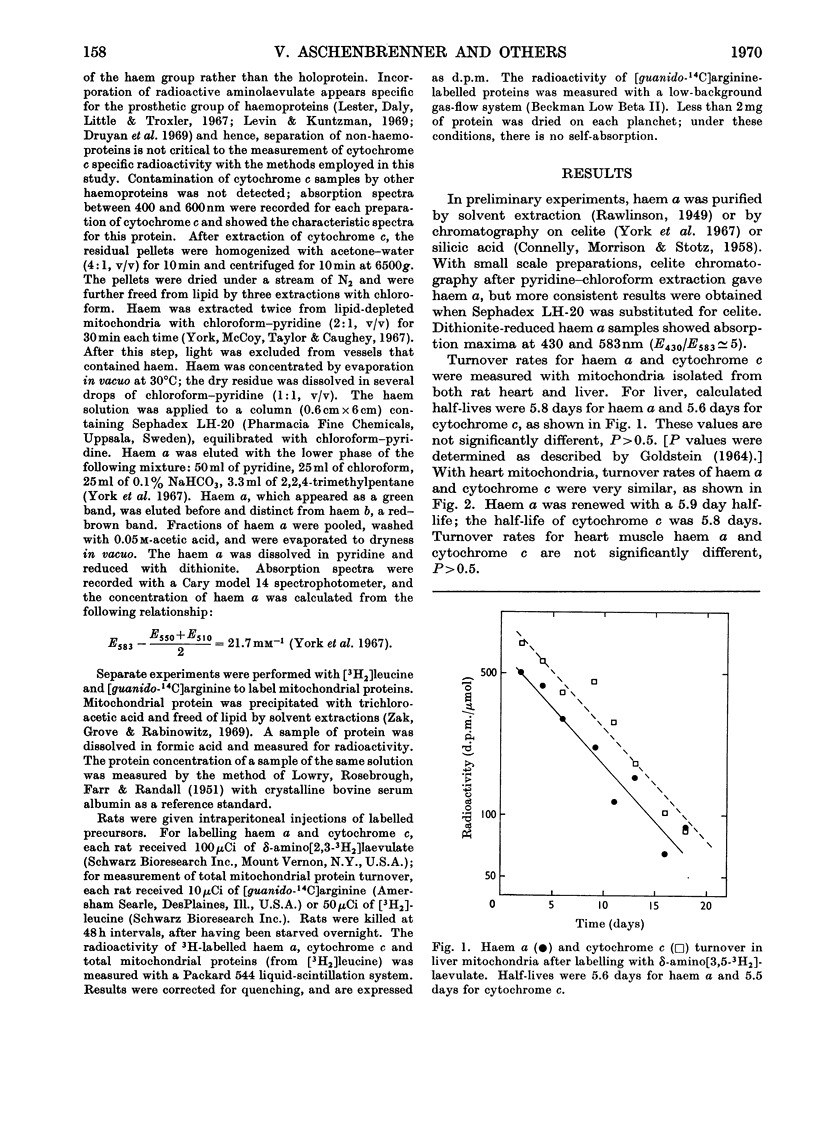
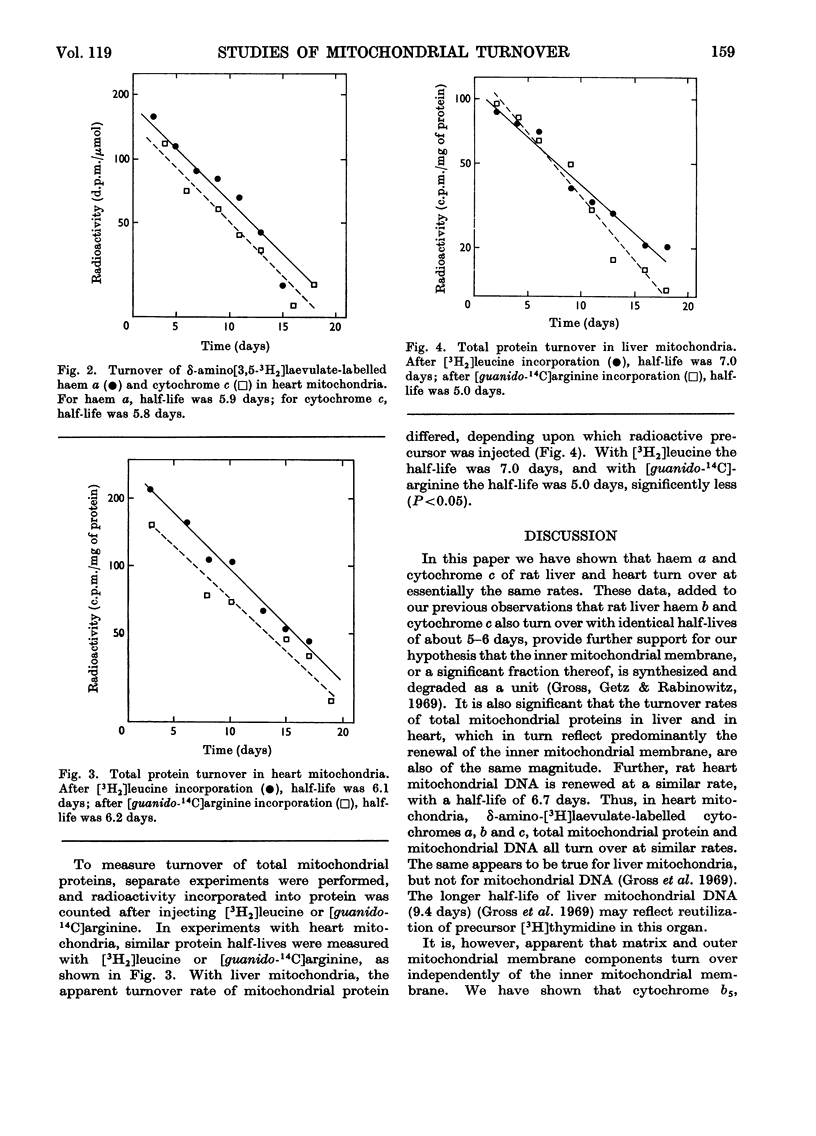
Haem a and cytochrome c were isotopically labelled in mitochondria from rat heart and liver after injection of δ-amino[2,3-3H2]laevulate, a specific haem precursor. [guanido-14C]Arginine or l-[4,5-3H2]leucine were used to label mitochondrial proteins. Half-lives were measured from biological decay in vivo and were similar (5.5–6.2 days) for haem a, cytochrome c and [14C]arginine-labelled proteins. Labelling of hepatic mitochondrial proteins with [3H2]leucine resulted in a prolonged apparent half-life.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie D. S. The turnover of the protein components of the inner and outer membrane fractions of rat liver mitochondria. Biochem Biophys Res Commun. 1969 Jun 6;35(5):721–727. doi: 10.1016/0006-291x(69)90465-3. [DOI] [PubMed] [Google Scholar]
- Brunner G., Neupert W. Turnover of outer and inner membrane proteins of rat liver mitochondria. FEBS Lett. 1968 Aug;1(3):153–155. doi: 10.1016/0014-5793(68)80045-6. [DOI] [PubMed] [Google Scholar]
- CONNELLY J. L., MORRISON M., STOTZ E. Hemins of beef heart muscle. J Biol Chem. 1958 Sep;233(3):743–747. [PubMed] [Google Scholar]
- Druyan R., DeBernard B., Rabinowitz M. Turnover of cytochromes labeled with delta-aminolevulinic acid-3H in rat liver. J Biol Chem. 1969 Nov 10;244(21):5874–5878. [PubMed] [Google Scholar]
- FLETCHER M. J., SANADI D. R. Turnover of rat-liver mitochondria. Biochim Biophys Acta. 1961 Aug 5;51:356–360. doi: 10.1016/0006-3002(61)90177-9. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Getz G. S., Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969 Mar 25;244(6):1552–1562. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin W., Kuntzman R. Biphasic decrease of radioactive hemoprotein from liver microsomal CO-binding particles. Effect of 3-methylcholanthrene. J Biol Chem. 1969 Jul 10;244(13):3671–3676. [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N., WIENER E. A study of the cytochrome c haemochromogen. Biochem J. 1959 Mar;71(3):559–570. doi: 10.1042/bj0710559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R., Druyan R., Getz G. S., Rabinowitz M. Intramitochondrial localization of delta-aminolaevulate synthetase and ferrochelatase in rat liver. Biochem J. 1969 Sep;114(3):455–461. doi: 10.1042/bj1140455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAWLINSON W. A., HALE J. H. Prosthetic groups of the cytochromes present in Corynebacterium diphtheriae with especial reference to cytochrome a. Biochem J. 1949;45(3):247-55, pl. doi: 10.1042/bj0450247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF LIVER AND YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1964 Nov;239:3908–3914. [PubMed] [Google Scholar]
- Schimke R. T., Ganschow R., Doyle D., Arias I. M. Regulation of protein turnover in mammalian tissues. Fed Proc. 1968 Sep-Oct;27(5):1223–1230. [PubMed] [Google Scholar]
- Swick R. W., Rexroth A. K., Stange J. L. The metabolism of mitochondrial proteins. 3. The dynamic state of rat liver mitochondria. J Biol Chem. 1968 Jul 10;243(13):3581–3587. [PubMed] [Google Scholar]
- Tschudy D. P., Marver H. S., Collins A. A model for calculating messenger RNA half-life: short lived messenger RNA in the induction of mammalian delta-aminolevulinic acid synthetase. Biochem Biophys Res Commun. 1965 Dec 9;21(5):480–487. doi: 10.1016/0006-291x(65)90408-0. [DOI] [PubMed] [Google Scholar]
- York J. L., McCoy S., Taylor D. N., Caughey W. S. Heme A of cytochrome c oxidase. I. Isolation from bovine heart. J Biol Chem. 1967 Mar 10;242(5):908–911. [PubMed] [Google Scholar]
- Zak R., Grove D., Rabinowitz M. DNA synthesis in the rat diaphragm as an early response to denervation. Am J Physiol. 1969 Mar;216(3):647–654. doi: 10.1152/ajplegacy.1969.216.3.647. [DOI] [PubMed] [Google Scholar]


