Abstract
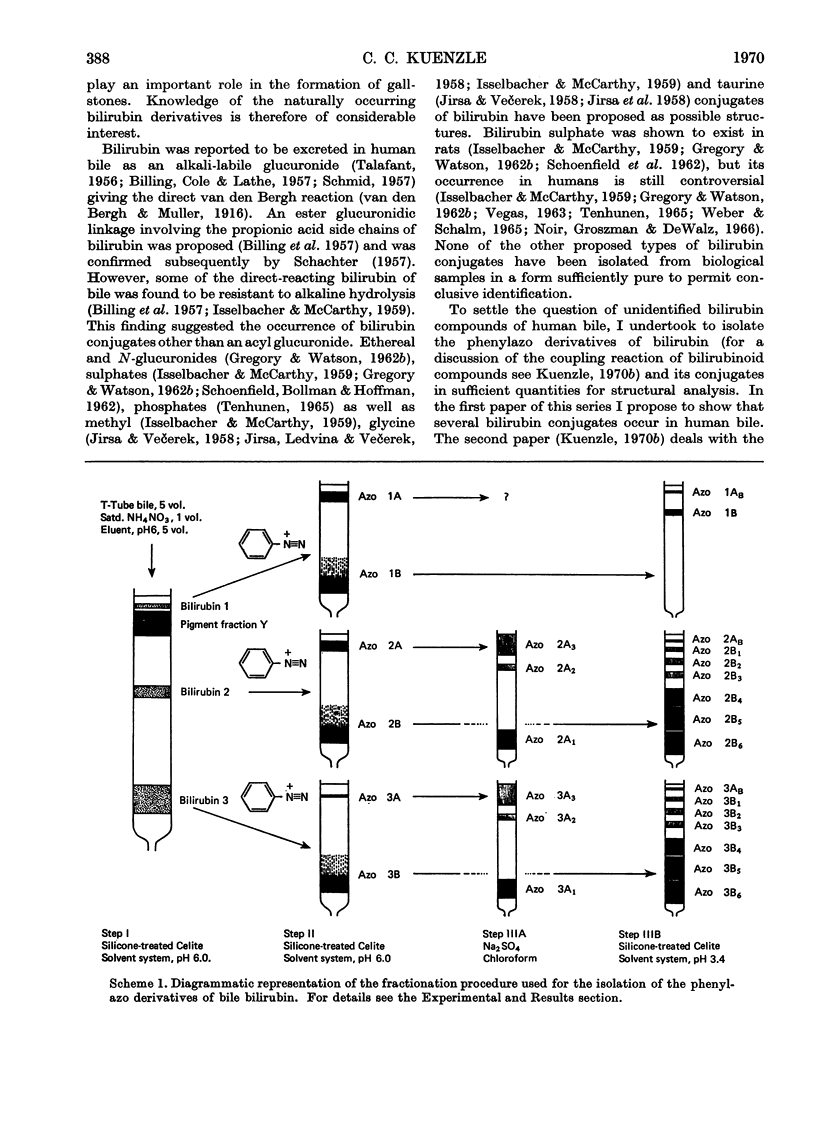
A method is presented that allows the isolation of eight different phenylazo derivatives of bile bilirubin. In step I of the isolation procedure, three bilirubin fractions (bilirubin fractions 1, 2 and 3) from human hepatic bile are separated by reverse-phase partition chromatography on silicone-treated Celite with the use of a solvent system prepared from butan-1-ol and 5mm-phosphate buffer, pH6.0. Azo coupling is then performed with diazotized aniline. The three azo pigment mixtures are subjected to step II, in which the above chromatography system is used again. With each azo pigment mixture this step brings about the separation of a non-polar and a polar azo pigment fraction (azo 1A and azo 1B, azo 2A and azo 2B, and azo 3A and azo 3B from bilirubin fractions 1, 2 and 3 respectively). Approximately equal amounts of non-polar and polar pigments are obtained from bilirubin fractions 1 and 2, whereas bilirubin fraction 3 yields azo 3B almost exclusively. In step IIIA the non-polar azo pigment fractions are fractionated further by adsorption chromatography on anhydrous sodium sulphate with the use of chloroform followed by a gradient of ethyl acetate in chloroform. Three azo pigments are thus obtained from both azo 2A (azo 2A1, azo 2A2 and azo 2A3) and azo 3A (azo 3A1, azo 3A2 and azo 3A3). The 2A pigments occur in approximately the following proportions: azo 2A1, 90%; azo 2A2, 10%; azo 2A3, traces. The pigments are purified by crystallization, except for the A3 pigments, which are probably degradation products arising from the corresponding A2 pigments. In step IIIB the polar azo pigment fractions are subjected to reverse-phase partition chromatography on silicone-treated Celite with the use of a solvent system prepared from octan-1-ol–di-isopropyl ether–ethyl acetate–methanol–0.2m-acetic acid (1:2:2:3:4, by vol.). Azo pigment fractions 2B and 3B each yield six azo pigments (azo 2B1 to azo 2B6 and azo 3B1 to azo 3B6 respectively) together with small amounts of products of hydrolysis (azo 2AB and azo 3AB). Only one azo B pigment is obtained from bilirubin fraction 1, and this azo pigment is probably of the B2 type. The yields of the azo 3B pigments suggest that these pigments are present in approximately the following proportions: azo 3B1, 0–0.4%; azo 3B2, traces; azo 3B3, traces; azo 3B4, 10%; azo 3B5, 50%; azo 3B6, 40%. Azo pigments 2B1 to 2B6 are estimated to occur in similar proportions. Since pairs of correspondingly numbered azo pigments from bilirubin fractions 1, 2 and 3 do not separate on rechromatography together (e.g. azo 2A1 co-chromatographs with azo 3A1, and azo 2B6 co-chromatographs with azo 3B6), it is concluded that such pigments are chemically identical. The structures of the isolated phenylazo derivatives are discussed in an accompanying paper (Kuenzle 1970c).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLING B. H., COLE P. G., LATHE G. H. The excretion of bilirubin as a diglucuronide giving the direct van den Bergh reaction. Biochem J. 1957 Apr;65(4):774–784. doi: 10.1042/bj0650774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY C. H. Studies of conjugated bilirubin. III. Pigment I, a complex of conjugated and free bilirubin. J Lab Clin Med. 1963 Jun;61:917–925. [PubMed] [Google Scholar]
- GREGORY C. H., WATSON C. J. Studies of conjugated bilirubin. I. Comparison of conventional fractional determination with chromatographic and solvent partition methods for free and conjugated bilirubin. J Lab Clin Med. 1962 Jul;60:1–16. [PubMed] [Google Scholar]
- GREGORY C. H., WATSON C. J. Studies of conjugated bilirubin. II. Problem of sulfates of bilirubin in vivo and in vitro. J Lab Clin Med. 1962 Jul;60:17–30. [PubMed] [Google Scholar]
- ISSELBACHER K. J., McCARTHY E. A. Studies on bilirubin sulfate and other nonglucuronide conjugates of bilirubin. J Clin Invest. 1959 Apr;38(4):645–651. doi: 10.1172/JCI103842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Nuclear-magnetic-resonance, infrared and optical spectra of model compounds. Biochem J. 1970 Sep;119(3):395–409. doi: 10.1042/bj1190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C., Maier C., Rüttner J. R. The nature of four bilirubin fractions from serum and of three bilirubin fractions from bile. J Lab Clin Med. 1966 Feb;67(2):294–306. [PubMed] [Google Scholar]
- Kuenzle C. C., Sommerhalder M., Rüttner J. R., Maier C. Separation and quantitative estimation of four bilirubin fractions from serum and of three bilirubin fractions from bile. J Lab Clin Med. 1966 Feb;67(2):282–293. [PubMed] [Google Scholar]
- Noir B. A., Groszman R. J., De Walz A. T. Studies on bilirubin sulphate. Biochim Biophys Acta. 1966 Apr 25;117(2):297–304. doi: 10.1016/0304-4165(66)90080-8. [DOI] [PubMed] [Google Scholar]
- SCHACHTER D. Nature of the glucuronide in direct-reacting bilirubin. Science. 1957 Sep 13;126(3272):507–508. doi: 10.1126/science.126.3272.507-a. [DOI] [PubMed] [Google Scholar]
- SCHALM L. [Research on the physiology of bilirubin]. Rev Int Hepatol. 1962;12:559–565. [PubMed] [Google Scholar]
- SCHMID R. The identification of direct-reacting bilirubin as bilirubin glucuronide. J Biol Chem. 1957 Dec;229(2):881–888. [PubMed] [Google Scholar]
- SCHOENFIELD L. J., BOLLMAN J. L., HOFFMAN H. N., 2nd Sulfate and glucuronide conjugates of bilirubin in experimental liver injury. J Clin Invest. 1962 Jan;41:133–140. doi: 10.1172/JCI104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALAFANT E. Properties and composition of the bile pigment giving a direct diazo reaction. Nature. 1956 Aug 11;178(4528):312–312. doi: 10.1038/178312a0. [DOI] [PubMed] [Google Scholar]
- VEGAS F. R. Chromatographic behavior on ion-exchange paper of bilirubin conjugated with sulfate and glucuronide. Anal Biochem. 1963 Jun;5:465–470. doi: 10.1016/0003-2697(63)90065-4. [DOI] [PubMed] [Google Scholar]


