Abstract
The septin proteins function in the formation of septa, mating projections, and spores in Saccharomyces cerevisiae, as well as in cell division and other processes in animal cells. Candida albicans septins were examined in this study for their roles in morphogenesis of this multimorphic, opportunistically pathogenic fungus, which can range from round budding yeast to elongated hyphae. C. albicans green fluorescent protein labeled septin proteins localized to a tight ring at the bud and pseudohyphae necks and as a more diffuse array in emerging germ tubes of hyphae. Deletion analysis demonstrated that the C. albicans homologs of the S. cerevisiae CDC3 and CDC12 septins are essential for viability. In contrast, the C. albicans cdc10Δ and cdc11Δ mutants were viable but displayed conditional defects in cytokinesis, localization of cell wall chitin, and bud morphology. The mutant phenotypes were not identical, however, indicating that these septins carry out distinct functions. The viable septin mutants could be stimulated to undergo hyphal morphogenesis but formed hyphae with abnormal curvature, and they differed from wild type in the selection of sites for subsequent rounds of hyphal formation. The cdc11Δ mutants were also defective for invasive growth when embedded in agar. These results further extend the known roles of the septins by demonstrating that they are essential for the proper morphogenesis of C. albicans during both budding and filamentous growth.
INTRODUCTION
The septins comprise a family of proteins that are involved in dynamic membrane events in a wide range of eukaryotic organisms (reviewed in Cooper and Kiehart, 1996; Longtine et al., 1996; Field and Kellogg, 1999; Trimble, 1999; Gladfelter et al., 2001; Kartmann and Roth, 2001). The majority of septin studies have been carried out in the budding yeast Saccharomyces cerevisiae, where four septins, Cdc3p, Cdc10p, Cdc11p, and Cdc12p, have been shown to make up a ring of 10-nm filaments on the inner surface of the plasma membrane at the bud neck (Byers and Goetsch, 1976a; Longtine et al., 1996; Frazier et al., 1998; Longtine et al., 1998). Temperature-sensitive mutants in the corresponding genes arrest with elongated buds that fail to undergo cytokinesis (Hartwell, 1971; Adams and Pringle, 1984). Consequently, these proteins became known as septins for their suspected role in forming the septum between the mother and daughter cells. Additionally, these mutants fail to form normal chitin rings in the cell wall at the bud neck and display a defect in the axial budding pattern (Slater et al., 1985; Flescher et al., 1993; Chant et al., 1995; DeMarini et al., 1997). The formation of the septin ring apparently requires interdependent interactions between the septin proteins. Shifting any of the septin mutants to the nonpermissive temperature resulted not only in the in the disappearance of the mutated septin but also of the other septins from the bud neck and the loss of the 10-nm filaments (Byers and Goetsch, 1976b; Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991). Deletion of CDC3 or CDC12 is lethal. Interestingly, deletion mutants lacking CDC10 or CDC11 are viable only at low temperatures and display phenotypes similar to the temperature-sensitive septin mutants (Flescher et al., 1993; Fares et al., 1996; Frazier et al., 1998). One additional bud neck-localized septin, Shs1p/Sep7p, was identified later; deletion of this gene caused mild defects in cytokinesis (Carroll et al., 1998; Mino et al., 1998).
The septins have also been implicated in morphogenetic processes other than budding in S. cerevisiae. In particular, septins have been shown to function in mating projection formation, where, similar to budding, the septins form a ring at the neck of the polarized growth that forms a conjugation bridge to connect the mating cells (Ford and Pringle, 1991; Kim et al., 1991; Giot and Konopka, 1997). The septins do not seem to form a tight ring structure, however, and 10-nm filaments have not been detected at the neck of mating projections. The septins were also implicated in sporulation by the observation that two S. cerevisiae septins, SPR3 and SPR28, are expressed only in cells undergoing meiosis and sporulation (Ozsarac et al., 1995; De Virgilio et al., 1996; Fares et al., 1996). Interestingly, the septins seem to be broadly distributed in the prospore membrane and are not restricted to a tight ring (De Virgilio et al., 1996; Fares et al., 1996; Tachikawa et al., 2001).
The high degree of homology between septin proteins, usually >26% identity over their entire length, has led to the identification of septins in a wide range of eukaryotic cells from yeast to human, with the exception of plants. Interestingly, septin proteins in multicellular organisms seem to function not only in cytokinesis (Neufeld and Rubin, 1994; Fares et al., 1995; Kinoshita et al., 1997; Nguyen et al., 2000) but also in other processes. For example, septins have been found to associate with the sec6/sec8 exocyst complex in rats (Hsu et al., 1998) and also with synaptic vesicles in mice (Beites et al., 1999), suggesting they might participate directly in vesicle trafficking and regulated secretion. Regardless of where and when the septins are expressed, however, they usually seem to be membrane associated and have been hypothesized to function as a network for organizing or regulating cellular events.
To better understand the roles of septins in diverse morphological processes, we investigated septin function in the pathogenic yeast Candida albicans. C. albicans was targeted for analysis because it is capable of switching from the round yeast form to other morphologies, including various elongated pseudohyphal forms and a highly elongated hyphal form that has not been observed in S. cerevisiae. The regulation of C. albicans morphogenesis is also significant because the ability to switch between budding and filamentous forms is an important virulence determinant for the pathogenicity of C. albicans. Septins were thought to be present in C. albicans because electron microscopy studies showed that 10-nm filaments could be observed at sites of septation (Soll and Mitchell, 1983). Subsequent DNA hybridization studies identified apparent homologs of the S. cerevisiae CDC3 and CDC10 genes (DiDomenico et al., 1994), and recently a green fluorescent protein (GFP) fusion of the C. albicans homolog of Cdc3p was localized to the bud neck (Gale et al., 2001; Gerami-Nejad et al., 2001). Antibodies against the S. cerevisiae Cdc11p also detected a putative C. albicans Cdc11p homolog that was present in budding, pseudohyphal, and hyphal growth phases (Sudbery, 2001). Therefore, we carried out an analysis of the seven septins that were identified by the C. albicans genome sequence. These studies indicate that in addition to their role in cytokinesis, septins function in guiding proper hyphal morphogenesis in C. albicans.
MATERIALS AND METHODS
Strains and Media
The C. albicans strains used in this study (Table 1) are derivatives of BWP17 (Wilson et al., 1999). The open reading frame (ORF) of each of the seven septins was deleted through successive transformations with polymerase chain reaction (PCR)-generated constructs that contained either ARG4 or HIS1 flanked by short regions of homology to the gene of interest (Wilson et al., 1999). The extents of the deletion mutations are as follows: for CDC3, codons 28–352 were removed, ORF is 378 codons; CDC10, Δ 26–464 of 489 codons; CDC11, Δ 28–376 of 402 codons; CDC12, Δ 27–365 of 370 codons; SEP7, Δ 32–583 of 615 codons; SPR3, Δ 35–458 of 491 codons; and SPR28, Δ 27–370 of 397 codons. Plasmid constructs for GFP-tagged septins or gene deletion restoration were introduced by targeted integrated at the particular septin locus, with the exception of CDC10-GFP constructs, which were integrated randomly into the genome.
Table 1.
C. albicans strains constructed for this study
| Strain | Parent | Genotype |
|---|---|---|
| BWP17a | Sc5314 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YAW1 | BWP17 | pAW-CDC10-GFP (integrated) |
| YAW2 | BWP17 | pAW-ADH-CDC10-GFP (integrated) |
| YAW4 | BWP17 | cdc10Δ::ARG4/CDC10 |
| YAW5 (cdc10Δ) | YAW4 | cdc10Δ::ARG4/cdc10Δ::HIS1 |
| YAW6 | YAW5 | cdc10Δ::ARG4/cdc10Δ::HIS1::CDC10-URA3 |
| YAW8 | BWP17 | cdc11Δ::ARG4/CDC11 |
| YAW9 (cdc11Δ) | YAW8 | cdc11Δ::ARG4/cdc11Δ::HIS1 |
| YAW10 | YAW9 | cdc11Δ::ARG4/cdc11Δ::HIS1::CDC11-URA3 |
| YAW12 | BWP17 | cdc12Δ::ARG4/CDC12 |
| YAW13 | BWP17 | cdc3Δ::ARG4/CDC3 |
| YAW14 | BWP17 | spr3Δ::ARG4/SPR3 |
| YAW15 (spr3Δ) | YAW14 | spr3Δ::ARG4/spr3Δ::HIS1 |
| YAW17 | BWP17 | spr28Δ::ARG4/SPR28 |
| YAW18 (spr28Δ) | YAW17 | spr28Δ::ARG4/spr28Δ::HIS1 |
| YAW20 | BWP17 | sep7Δ::ARG4/SEP7 |
| YAW21 (sep7Δ) | YAW20 | sep7Δ::ARG4/sep7Δ::HIS1 |
| YAW23 | YAW5 | cdc10Δ::ARG4/cdc10Δ::HIS1 cdc12::CDC12-GFP-URA3/CDC12 |
| YAW24 | YAW5 | cdc10Δ::ARG4/cdc10Δ::HIS1 cdc3::CDC3-GFP-URA3/CDC3 |
| YAW25 | YAW9 | cdc11Δ::ARG4/cdc11Δ::HIS1 cdc12::CDC12-GFP-URA3/CDC12 |
| YAW26 | YAW9 | cdc11Δ::ARG4/cdc11Δ::HIS1 cdc3::CDC3-GFP-URA3/CDC3 |
| YAW27 | YAW9 | cdc11Δ::ARG4/cdc11Δ::HIS1 pAW-CDC10-GFP (integrated) |
| YAW28 | YAW9 | cdc11Δ::ARG4/cdc11Δ::HIS1 pAW-ADH-CDC10-GFP (integrated) |
| YAW44 | BWP17 | cdc12::CDC12-GFP-URA3/CDC12 |
| YAW45 | BWP17 | cdc3::CDC3-GFP-URA3/CDC3 |
The C. albicans strains were propagated on either YPD or minimal defined medium (Sherman, 1991) plus 50 mg/l uridine unless indicated otherwise. Hyphal growth was induced by the addition of 20% bovine calf serum (BCS) to YPD, or in Lee's medium (Buffo et al., 1984). For agar-embedded hyphal assays, Spider medium (10 g of Bacto yeast nitrogen base, 10 g of mannitol, 2 g of K2HPO4, and 13.5 g of agar per liter), GlcNAc (6.7 g of Bacto yeast nitrogen base, 2.5 mM N-acetyl-d-glucosamine, 15 g of agar per liter), and 4% serum agar (40 ml of BCS, 15 g of agar per liter) were all supplemented with 20 mg/l histidine, 20 mg/l arginine, and 80 mg/l uridine.
Primers, Plasmids, and PCR
All primer sequences are listed in Table 2 as online supplemental material. pAW-CDC10-Res, used to restore a single copy of CDC10 to the cdc10 deletion strain, was constructed by amplifying from BWP17, a 1.8-kb segment of CDC10 flanked by AatII and SacII ends by using primers 5′CDC10-RESTORE and 3′CDC10-RESTORE and ligating into pGEM-URA3 (Wilson et al., 1999). pAW-CDC11-Res, for restoration of CDC11 to the cdc11 deletion strain, was created essentially the same way from a 2.5-kb segment of CDC11. pAW-CDC10-GFP, used to generate GFP-tagged Cdc10p from the endogenous CDC10 promoter, was constructed from pUC19 as follows. GFP was amplified from pUC19 containing yEGFP (Cormack et al., 1997) with SacI and EcoRI ends from primers GFP3–5′ and GFP3–3′ and cloned into pUC19. A 1.4-kb fragment of URA3 was amplified by primers URA3–5′ and URA3–3′ from Sc5314 (Fonzi and Irwin, 1993) and cloned into the GFP-containing pUC19 by using AatII sites provided by the primers, resulting in plasmid pAW-REMI. A 1.5-kb segment of CDC10 was then amplified from BWP17 with primers 5′CDC10-BAM and 3′CDC10-ORF, which provided BamHI and SacI sites to clone CDC10 in frame with GFP. pAW-CDC12-GFP was created similarly. Primers 5′CDC12-BAM and 3′CDC12-ORF were used to amplify a 1.6-kb segment of CDC12 from BWP17 with BamHI and SacI ends, which was then ligated into pAW-REMI. A similar plasmid with CDC3 in frame with GFP was also constructed (Martin and Konopka, unpublished data). pAW-ADH-CDC10-GFP, for expression of GFP-tagged Cdc10p from the constitutive overexpression promoter ADH1, was created by amplifying GFP from pAW-REMI with primers 5′GFP-XHO and 3′GFP-MLU and ligation of the GFP into the URA3 plasmid YPB1-ADHpt (Bailey et al., 1996) at MluI and XhoI sites. A BamHI-SacI fragment containing the CDC10 ORF was then generated by PCR of BWP17 DNA with 5′CDC10-ORF and 3′CDC10-ORF and ligated into the ADH-GFP plasmid at its BglII and SacI sites.
To perform gene disruptions, PCR mixtures contained ∼10 ng of alkaline lysis prep DNA (pGEM-URA3, pGEM-HIS1, or pRS-ARG4ΔSpeI for gene deletion [Wilson et al., 1999] or pBME101 for the test of gene essentiality [Enloe et al., 2000]) as template, 0.2 μm each dNTP, 1× PCR buffer minus Mg2+ (Invitrogen, Carlsbad, CA), 1 mM magnesium chloride, 40 pmol of each primer, 50 ng of bovine serum albumin, and 2.5 U of Taq DNA polymerase in a 100-μl reaction. Amplification was performed as follows: 94°C for 5 min followed by 25 cycles of 93°C for 30 s, 53°C for 60 s, and 72°C for 3 min. Primers used for the generation of disruption constructs are labeled with DR or FIX extensions (see online supplemental Table 2). The DR series bears homology to both the plasmids and the target gene, and the FIX series pair to the last few bases of the corresponding DR primers to ensure that the resulting PCR product is completely amplified. Primers labeled with ORF, DET, and UNIV extensions were used for the detection of the gene disruptions with ARG4, HIS1, or UAU1 (test of gene essentiality) by using bead-bashed genomic DNA (Hoffman and Winston, 1987) as template and the same reaction conditions described above.
C. albicans Transformations
Transformations were performed essentially as described previously (Wilson et al., 1999). Briefly, overnight cultures were diluted 1:500 in 100 ml of medium and grown ∼8 h at 30°C to an OD660 of 0.8–1.0. Cells were harvested by centrifugation, washed in 1/10 volume LATE buffer (0.1 M lithium acetate, 10 mM Tris-HCl pH 7.5, and 1 mM EDTA) and resuspended in 1/50 volume of LATE buffer. Then 100 μl of the cell suspension was mixed with 50 μg of sheared salmon sperm DNA and either 95 μl of a disruption construct PCR reaction or 5 μg of linearized plasmid DNA. Polyethylene glycol 4000 (40%) in LATE buffer was mixed into the reaction, which was then incubated at 30°C for 12–15 h. The cells were heat shocked for 1 h at 42°C, pelleted, and washed in YPD medium before being resuspended in 200 μl of YPD and plated on selective medium.
Microscopy
Cells were examined using differential interference contrast optics (DIC) for morphological analysis or fluorescence microscopy to visualize GFP, 4,6-diamidino-2-phenylindole (DAPI), or Calcofluor. GFP localization and analysis were performed on cultures in logarithmic growth without fixation (unless DAPI staining was necessary) under the fluorescein isothiocyanate channel. DAPI staining was performed on formaldehyde-fixed cells by using 5 μg/ml in Prolong Antifade mounting medium (Molecular Probes, Eugene, OR) and viewed under UV. Fixation did not exceed 15 min in cells where visualization of GFP was also necessary, but typically ranged from 1 to 3 h for DAPI alone. Chitin staining was performed by adding 0.2 μg/ml Calcofluor White directly to the medium during the last 10–20 min of growth and viewing the cells without fixation under UV. Actin staining was performed on formaldehyde-fixed cells by using 5 U/sample rhodamine phalloidin (Molecular Probes). All microscopy was performed on an Olympus BH-2 microscope. Images were captured with a black-and-white AxioCam (Carl Zeiss, Thornwood, NY) run by Openlab 3.0.1 software from Improvision (Lexington, MA). Image pseudocoloring and merging were performed using the Openlab 3.0.1 software. For quantitation of phenotypes, at least 100 (but more commonly 300–800) cells were counted from two to five independent experiments to generate the percentages reported.
RESULTS
Seven Septin Genes in C. albicans
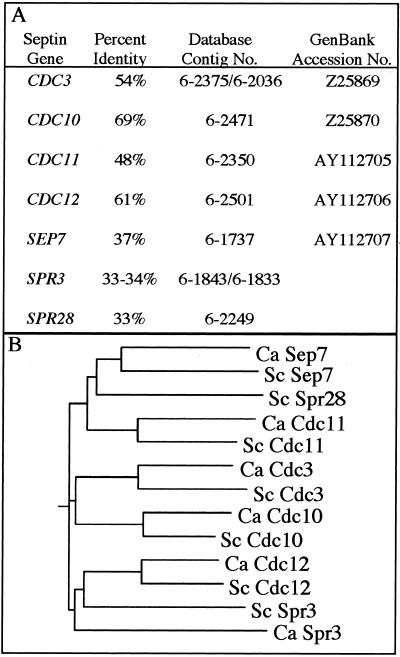
Seven septin genes are known in S. cerevisiae: CDC3, CDC10, CDC11, CDC12, SEP7, SPR3, and SPR28 (see INTRODUCTION). Sequences homologous to all seven were found in C. albicans by using a tBLASTn search against genomic sequence data and are listed in Figure 1A. Comparison of the predicted proteins showed that sequence conservation was greatest for the four septins involved in cytokinesis in S. cerevisiae (Cdc3p, Cdc10p, Cdc11p, and Cdc12p), which ranged from 48 to 69% identity in sequence to the C. albicans homolog. The relationship for the sporulation-specific septins Spr3p and Spr28p was somewhat lower at 33% identity. A multiple sequence alignment was used to construct a phylogenetic tree that confirmed the relatedness of the protein sequences (Figure 1B). The C. albicans sequence showing homology to S. cerevisiae Spr28p seems to contain a shift in reading frame between the regions of similarity and thus was omitted from the tree analysis.
Figure 1.
C. albicans septins. (A) Homologs of the seven known septin genes from S. cerevisiae were identified using a tBLASTn search against the C. albicans genome sequence (Stanford Genome Technology Center Web site at http://www-sequence.stanford.edu/group/candida). Amino acid identities as reported by the search are indicated. (B) Phylogenetic tree of the S. cerevisiae and C. albicans sequences was generated using the CLUSTAL W program at http://workbench.sdsc.edu.
Distinct Septin Localization during Budding, Pseudohyphal, and Hyphal Growth
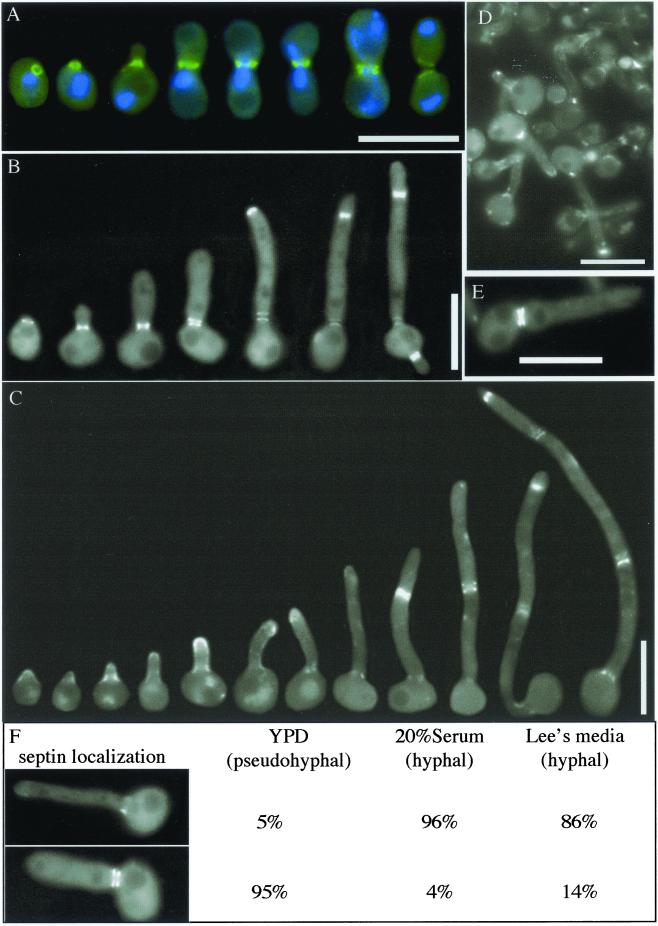
A CDC10-GFP fusion was integrated into wild-type strain BWP17 to observe subcellular septin localization during budding, pseudohyphal, and hyphal growth stages. Because fluorescence was weak in the early stages of hyphal growth, the GFP fusion was also expressed from the ADH1 promoter to enhance visibility. The pattern of Cdc10-GFP localization, both spatially and temporally, was similar in cells expressing the fusion gene from the CDC10 or ADH1 promoter (our unpublished data). In addition, overexpression of the septin-GFP fusion did not cause any apparent aberrant phenotype under any conditions tested.
The overall pattern of septin localization observed during budding in C. albicans was similar to what has previously been seen in S. cerevisiae (Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991). As shown in Figure 2A, cells grown under conditions that promote budding exhibited septin-GFP localization as a tight ring that appeared just before bud emergence. The bud then grew out through the septin ring, which appeared as a doublet around the time of nuclear division. Shortly after the ring structure divided, the GFP decreased in intensity, indicating breakdown of the septin ring, concurrent with mother/daughter cell separation. Similar results were obtained with strains expressing Cdc3-GFP and Cdc12-GFP (see below; Figures 4 and 5). These data, together with previous analysis of Cdc3p (Gale et al., 2001; Gerami-Nejad et al., 2001) and Cdc11p (Sudbery, 2001), indicate that C. albicans septins are conserved in localization as well as in sequence with the S. cerevisiae septins.
Figure 2.
Septin localization in budding, pseudohyphal, and hyphal cells. (A) Wild-type cells carrying an integrated ADH1-CDC10-GFP construct (YAW2) were grown to log phase at 30°C, fixed in 5% formaldehyde for 10 min, washed, and resuspended in mounting medium containing 5 μg/ml DAPI. Cells were photographed through a fluorescence microscope and then the images were pseudocolored (blue, DNA; green, septin) and merged using Openlab 3.0.1 software. Representative cells were chosen to show the progression of ring dynamics over time. (B) Pseudohyphal induction of stationary phase YAW2 cells diluted to 106/ml in YPD was carried out at 37°C. Cells were collected and photographed without fixation 1–3 h postinduction, and representative cells were chosen to show the progression of septin localizationin pseudohyphae over time. (C–E) Hyphal induction of stationary phase YAW2 cells diluted to 106/ml in medium containing 20% BCS was carried out at 37°C. Cells were collected between 30 min and 3 h postinduction, washed in phosphate-buffered saline, and photographed without fixation. (C) Representative cells were chosen to show the progression of septin localization in hyphae over time. (D) Corresponding image of a field of randomly selected cells 1 h postinduction. (E) Septin localization in a budding cell induced with serum to form a germ tube. (F) Patterns of septin localization observed at the necks of emerging growths were quantitated for 400–800 cells of strain YAW2 in one pseudohyphal-inducing and two hyphal-inducing conditions at 37°C, 1 h postinduction. Bars, 10 μm.
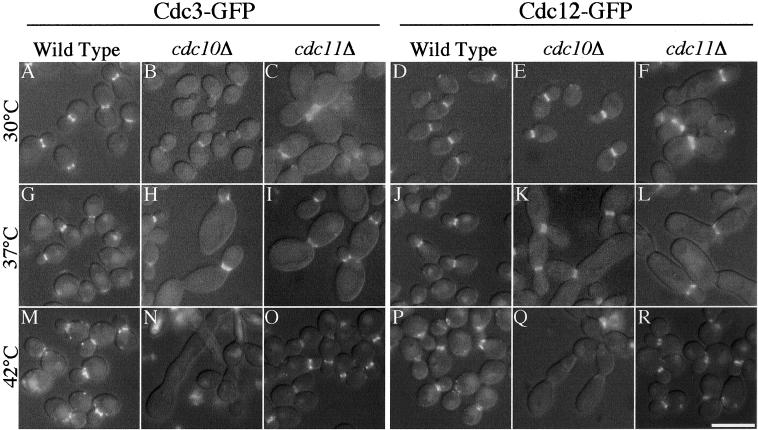
Figure 4.
Septin mutants assemble septin rings during yeast-form growth. Wild-type strains YAW44 (D, J, and P) and YAW45 (A, G, and M), cdc10Δ strains YAW23 (E, K, and Q) and YAW24 (B, H, and N), and cdc11Δ strains YAW25 (F, L, and R) and YAW26 (C, I, and O), all containing CDC3-GFP or CDC12-GFP integrated under control of the endogenous promoter, were grown for 12 h in log phase at the indicated temperature. Fluorescence and DIC images were captured and merged using Openlab 3.0.1 software. Bar, 10 μm.
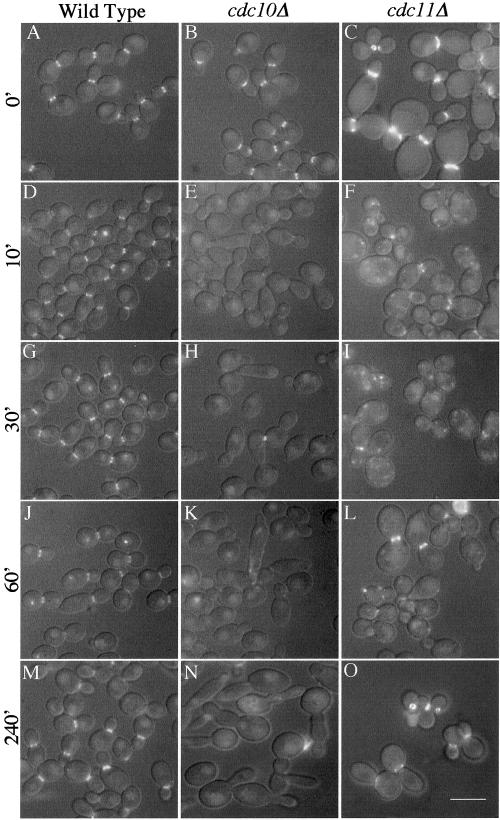
Figure 5.
Septin mutants show septin ring destabilization when shifted to 42°C. Wild-type strain YAW44 (A, D, G, J, and M), cdc10Δ strain YAW23 (B, E, H, K, and N), and cdc11Δ strain YAW25 (C, F, I, L, and O), all carrying an integrated CDC12-GFP under control of the endogenous promoter, were grown at 30°C for 24 h at low density and then shifted to 42°C. Aliquots of cells were removed at various times and photographed immediately without fixation. Fluorescence and DIC images were captured and merged using Openlab 3.0.1 software. Bar, 10 μm.
A similar pattern of neck ring localization of septins was observed in cells induced to undergo pseudohyphal growth (Figure 2B). Pseudohyphae are described as chains of connected buds that can vary in shape from small round cells to highly elongated cells, but that all bear distinct constrictions at septation sites (Odds, 1988). Just as in budding, pseudohyphal cells displayed a ring of Cdc10-GFP from which an elongated bud structure emerged. A similar observation was made by immunostaining with anti-Cdc11p antibody (Sudbery, 2001). However, as the pseudohyphae continued to elongate, new septin rings were detected at the growing tip in intervals of 10–15 μm from older rings, which were still present toward the base. This differed from budding cells in which old rings disassembled before the formation of new ones. Older rings in the pseudohyphae eventually appeared as doublets, coincident with the appearance of divided nuclei on either side, and then a septum formed between the rings (our unpublished data). After septation, the septin rings began to fade, and the mother cells could be separated easily by mechanical force.
The pattern of septin localization in the emerging germ tube of cells induced to undergo hyphal growth differed from that of budding or pseudohyphal cells. A faint mesh or cap-like structure was observed initially at the site of germ tube emergence, not the distinct ring observed for the other growth forms (Figure 2, C and D). This differs from a previous report of a ring that was observed by immunostaining with antibody against the S. cerevisiae Cdc11p (Sudbery, 2001). As the germ tube elongated, the Cdc10-GFP at the neck continued to appear more diffuse and less intense than the rings observed in buds or pseudohyphae. Faint, diffuse GFP fluorescence could also be observed at the tip in the early stages of germ tube growth. The cells displaying this pattern of septin localization corresponded to true hyphae because they lacked the distinct constriction at the neck that was observed for pseudohyphae, instead displaying either a straight or conical attachment to the mother cells.
Later in the hyphal growth, the Cdc10-GFP appeared as a tight ring structure in the germ tube 10–15 μm from the mother cell, at a site that marked the position of subsequent septum formation (Figure 2C, right). Similar results have been reported for Cdc3p and Cdc11p (Gale et al., 2001; Sudbery, 2001). Nuclear division occurred across the septin ring, which then appeared as a doublet coincident with a visible septum observable by DIC microscopy and a band of chitin observed by Calcofluor staining (our unpublished data). As the hypha continued to elongate by tip growth, a new ring appeared 10–20 μm from the previous one. Nuclear division ensued, one nucleus migrated across the ring, and then a septum formed. This pattern continued along the length of the hypha. The nucleus from the mother cell always returned to the main body of the mother cell, and nuclei from hyphal compartments ultimately came to rest in the center of each cell (our unpublished data; Sudbery, 2001). Interestingly, older septin rings disassembled slowly, especially on the mother cell side of the doublet. Septin rings in various stages of assembly, division, and disassembly could be observed simultaneously along the length of a single hypha. In contrast to pseudohyphae, hyphal cells were not easily separated.
The distinct septin patterns seemed likely to be useful for rapid discrimination between hyphae and pseudohyphae in a population of cells. Indeed, CDC10-GFP cells grown under pseudohyphal-inducing conditions displayed primarily (95%) the expected tight rings of septins that formed an apparent doublet at the neck (Figure 2F). In contrast, cells induced with serum showed primarily the hyphal pattern of diffuse neck fluorescence. A few of the serum-induced cells showed the pseudohyphal pattern, but most of these are likely to correspond to cells that were budding when induced. Budding cells evaginate the germ tube from the growing bud instead of the mother cell (our unpublished data; Hazan et al., 2002). The budding cells maintained the septin rings that had formed for bud emergence, but no ring structure was observed where the hypha emerged from the bud (Figure 2E). Not counting cells with obvious bud swellings at the base of the hypha, ∼4% of the serum-induced cells still seemed to have a septin ring that was reminiscent of budding or pseudohyphal cells. This was either due to a small bud having been converted into a hypha or to a low percentage of pseudohyphal growth in the serum conditions. In contrast, cells grown in Lee's medium to induce hyphal growth showed 86% hyphal pattern and 14% pseudohyphal pattern. The lower efficiency of hyphal induction in Lee's medium is consistent with serum being one of the strongest known inducers of hyphal growth.
Deletion of Septins Results in Aberrant Budding Phenotypes
Deletion analysis (see MATERIALS AND METHODS) was used to determine which septins are important for growth and morphogenesis in C. albicans. Individual septin open reading frames were disrupted by replacement of the septin coding sequences with ARG4 or HIS1 in the auxotrophic strain BWP17 (Wilson et al., 1999). Deletion of SEP7, SPR3, or SPR28 resulted in no observable phenotype under any conditions tested. This is consistent with reverse transcription-PCR results in which the expression of these sequences was not detectable during any growth phase (our unpublished data). In contrast, deletion of CDC10 or CDC11 caused strong phenotypes, and CDC3 and CDC12 seem to be essential genes.
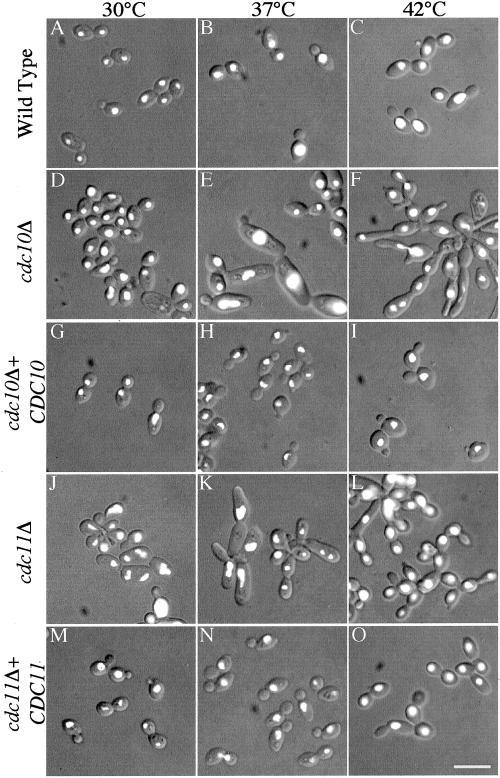
The majority of cdc10Δ cells appeared to be wild type when grown at 30°C (Figure 3D), but when shifted to 37°C, 34% of the cells were enlarged and elongated, and often remained attached end to end in a pseudohyphal-like pattern (Figure 3E). At 42°C, 94% of the cells became even more elongated, and many buds formed as club-like structures (Figure 3F). Interestingly, the mutants did not show an overall growth rate defect, even though the elongated, connected buds could not generally be separated by zymolyase digestion, indicating a frequent failure of cytokinesis. This contrasts with the S. cerevisiae cdc10Δ mutant, which exhibits slow growth and lysis (Flescher et al., 1993; Fares et al., 1996; DeMarini et al., 1997; Frazier et al., 1998). The phenotype of the cdc10Δ C. albicans cells was corrected by reintroduction of CDC10 or CDC10-GFP under control of the endogenous promoter as well as by CDC10-GFP overexpressed from the ADH1 promoter (Figure 3, G–I; our unpublished data).
Figure 3.
Septin mutants display altered cell morphology. C. albicans strains analyzed included wild-type strain BWP17 (A–C), homozygous deletion strain cdc10Δ YAW5 (D–F), and homozygous deletion strain cdc11Δ YAW9 (J–L). In addition, cdc10Δ or cdc11Δ strains carrying a single copy of the corresponding wild-type gene integrated under control of the endogenous promoter (YAW6; G–I) and (YAW10; M–O) were analyzed to confirm that the phenotype was due to the indicated gene deletion. Cells were grown at the indicated temperature for 12 h, fixed for 1 h in 5% formaldehyde, and stained with 0.5 μg/ml DAPI. Fluorescence and DIC images were acquired and merged using Openlab 3.0.1 software. Bar, 10 μm.
The cdc11Δ mutant exhibited a phenotype that was observable at any temperature. Colonies on a plate appeared rough and asymmetrical compared with the smooth, waxy appearance of wild-type or cdc10Δ colonies. In liquid culture at 30°C, 91% of the cdc11Δ cells proved to be abnormal; they varied in size and were often found growing in clusters that suggested a failure of separation of the buds from a mother cell exhibiting an axial budding pattern (Figure 3J). At elevated temperatures (Figure 3, K and L), the cells appeared to grow in both axial clusters of buds as well as the end-to-end polar budding arrangement observed for cdc10Δ mutants. Unlike the cdc10Δ cells, however, cdc11Δ mutants mainly formed round cells and generally did not display elongated buds at 42°C (Figure 3L). Like the cdc10Δ mutant, the cdc11Δ cells were generally nonseparable, yet were easily propagated at all temperatures despite their phenotype. The mutant phenotype was complemented by integration of a single copy of CDC11 under control of the endogenous promoter (Figure 3, M–O), but not overexpressed CDC10 (our unpublished data).
Both the cdc10Δ and cdc11Δ mutants displayed multiple nuclei in the enlarged cells or compartments, further indicating that the defect is in cell separation, not the cell cycle itself. Actin localization also appeared normal; cells displayed punctate patches in growing buds and filamentous actin cables in mother cells (our unpublished data).
Homozygous deletion mutants of CDC3 and CDC12 could not be isolated, suggesting that they may be essential, as are the homologs in S. cerevisiae. Therefore, a gene essentiality test was performed (Enloe et al., 2000). Briefly, a UAU1 (ura3-ARG4-ura3) construct was used to delete one target gene allele. Medium lacking arginine and uracil was then used to select for cells that had undergone two recombination events: 1) replacement of the second allele by the UAU1 construct and 2) a subsequent popout event at one UAU1 that results in a functional URA3 gene. However, for both CDC3 and CDC12, all candidate colonies (derived from three independent UAU1 deletion strains in each case) still contained a wild-type copy of the gene, presumably due to triploidy of the relevant chromosome (Enloe et al., 2000), indicating that both CDC3 and CDC12 are indeed essential.
Septin Mutants Form Unstable Septin Rings
The localizations of two other septins were examined in the cdc10 and cdc11 deletion mutants by integrating GFP-tagged CDC3 and CDC12 under the control of their endogenous promoters. In S. cerevisiae, the septin ring is typically disrupted when one septin is mutated or deleted (see INTRODUCTION), but this is not always the case in C. albicans. At 30 and 37°C, both the cdc10Δ and cdc11Δ mutants showed visible septin ring structures at the necks of growing buds (Figure 4, B, C, E, F, H, I, K, and L). However, when grown at 42°C for 12–15 h, cdc10Δ strains did not (<3%) show detectable Cdc3-GFP (Figure 4N) or Cdc12-GFP (Figure 4Q) localization. In contrast, the cdc11Δ mutants clearly displayed septin rings at the expected positions in the majority of cells (91%) at 42°C (Figure 4, O and R; our unpublished data for Cdc10-GFP). However, these rings sometimes appeared more punctate or diffuse than at lower temperatures.
A time course was conducted to analyze the behavior of the septin rings in the cdc10Δ and cdc11Δ mutants after a shift from 30 to 42°C. Within 10 min, the localized Cdc12-GFP signal was dramatically reduced in the cdc10Δ mutant (Figure 5E), and by 30 min only 1% of the cells displayed a visible septin ring, and these rings were markedly reduced in intensity (Figure 5H). The septin rings did not reappear upon further incubation (Figure 5, K and N). Surprisingly, cdc11Δ cells also displayed a dramatic reduction in the frequency and intensity of Cdc12-GFP rings during the first 30 min after the shift to 42°C (Figure 5, F and I). The loss of the rings was accompanied by an increase in punctate GFP localization in the cytoplasm, a pattern not seen in the cdc10Δ or wild-type cells (compare Figure 5, G–I).
On extended incubation at 42°C, the cdc11Δ cells recovered Cdc12-GFP rings, concomitant with a loss of the cytoplasmic GFP speckles (Figure 5, L and O; see also Figure 4, O and R). By 4 h the majority of the cdc11Δ cells had visible rings at the junction of new bud growth. Similar results were observed with Cdc3-GFP in both mutants and with Cdc10-GFP in cdc11Δ cells (our unpublished data). Thus, the absence of Cdc10p results in a complete defect in ring maintenance and assembly at 42°C, whereas absence of Cdc11p results in an initial reduction in septin ring stability upon shift to 42°C. However, rapid acclimation to the new growth temperature allows septin rings to reform in the absence of Cdc11p.
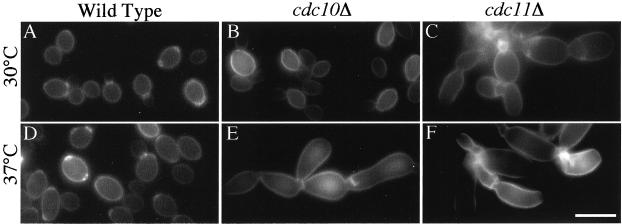
Septin Mutants Display Aberrant Chitin Localization
Despite their formation of septin rings, cdc10Δ and cdc11Δ strains are not normal for chitin localization. Wild-type cells displayed chitinous bud scars, detectable by Calcofluor staining, at the poles (Figure 6, A and D). In contrast, cdc10Δ cells, despite their apparently normal budding at 30°C, did not exhibit visible bud scars (Figure 6B). At 37°C, where their septin defect is more apparent, the cdc10Δ cells displayed a lack of septum formation between cell compartments and/or a mislocalization of chitin within growing compartments (Figure 6E). These phenomena were even more apparent in the cdc11Δ cells grown at 30 or 37°C (Figure 6, C and F). These results indicate that the septin rings that form in the absence of Cdc10p or Cdc11p are not sufficient to correctly localize the chitin synthase machinery.
Figure 6.
Septin mutants display aberrant chitin localization. Wild-type strain BWP17 (A and D), cdc10Δ strain YAW5 (B and E), and cdc11Δ strain YAW9 (C and F) were grown overnight at 30 or 37°C. The cells were then stained for 1 h with 0.2 μg/ml Calcofluor White, washed, and photographed. Bar, 10 μm.
Septin Mutants Show Altered Germ Tube and Hyphal Growth Patterns on a Solid Substrate
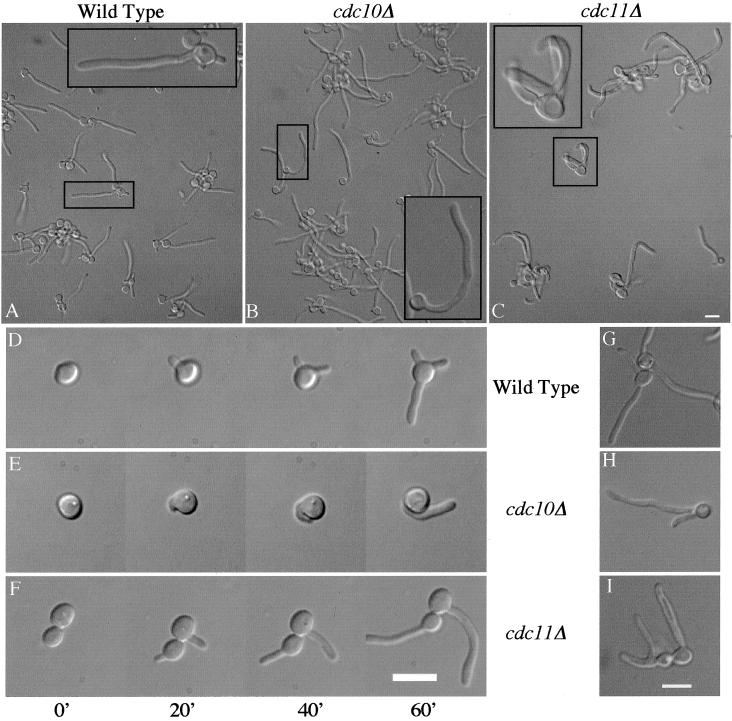
Because budding morphology was altered in the cdc10Δ and cdc11Δ mutants at 37°C, the temperature at which filamentation takes place, we examined whether these mutants would also display defects in hyphal growth. By 2.5 h postinduction, the majority of both wild-type (Figure 7A) and mutant (Figure 7, B and C) cells displayed significant germ tube or hyphal growth. However, the mutant strains displayed several characteristics that distinguished them from the wild type. The hyphae on wild-type cells generally grew out perpendicular to the parental cell and tracked straight across the surface of the agar (Figure 7A). In contrast, the majority of the septin mutant cells showed various degrees of curvature along the length of the hyphae, with 48–52% exhibiting a >45° curve compared with 12% for wild type (Figure 7, B and C). These curves originated either at the site of germ tube emergence or further out in the germ tube.
Figure 7.
Septin mutants show altered germ tube growth patterns. Wild-type strain BWP17 (A, D, and G), cdc10Δ strain YAW5 (B, E, and H), and cdc11Δ strain YAW9 (C, F, and I) were grown at 30°C overnight and then plated at low density on solid agar medium containing 20% BCS at 37°C. A high degree of filamentation had occurred within 2.5 h and was photographed (A–C, insets show an enlargement of the boxed cell). (D–F) Time-lapse series of photographs of a cell undergoing serum induction for 0, 20, 40, and 60 min. Secondary filamentation was apparent at 3–4 h (G, H, and I). Bars, 10 μm.
Interestingly, wild-type cells displayed a switching phenomenon that was captured during time-lapse photography of the serum induction. Early in the induction, cells would often begin and then abort one or more short germ tubes before finally extending the last projection into elongated hyphal growth (Figure 7D). In contrast, the septin mutants displayed this phenomenon less frequently and only rarely formed the multiple protruberances that were commonly observed in wild-type cells (Figure 7, E and F). At later times, wild-type cells formed a second hypha at a seemingly random position relative to the original hypha, with only 15% displaying the second hypha adjacent to the first (Figure 7G). In contrast, both septin mutants usually launched the second hypha from a position proximal to the first (62–74%) and often directly adjacent to it (Figure 7, H and I).
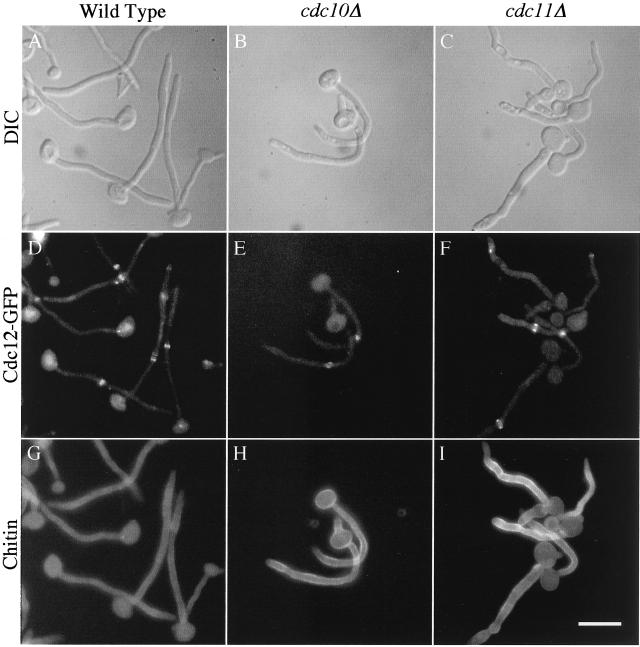
To investigate further the different growth patterns of the wild-type and mutant hyphae, we examined septin and chitin localization. Cdc12p localization at early times of induction for both mutant and wild-type cells was inconclusive due to a weak signal and high background in serum-containing media. Later, when the germ tubes septated, Cdc12-GFP rings were clearly visible along the growing hyphae in both the wild-type (Figure 8D) and mutant strains (Figure 8, E and F). Similar results were observed with Cdc3-GFP in both mutants and Cdc10-GFP in cdc11Δ cells. Thus, as in budding cells, hyphal cdc10Δ and cdc11Δ cells were not detectably defective in localization of the other septins during growth at 37°C. Also, as in budding cells, the mutants showed abnormal chitin localization (Figure 8, H and I), often displaying more irregular deposition in the wall as compared with wild type (Figure 8, G–I). This staining also revealed that the walls of the cdc11Δ hyphae, in particular, were not as smooth or parallel as the wild type. Taken together, the results indicate that the septin mutants are not globally defective for serum-induced hyphal growth, but they carry out this morphogenesis in an altered pattern compared with wild-type cells.
Figure 8.
Septin mutants assemble septin rings during hyphal growth. Wild-type strain YAW44 (A, D, and G), cdc10Δ strain YAW23 (B, E, and H), and cdc11Δ strain YAW25 (C, F, and I), all carrying CDC12-GFP integrated under control of the endogenous promoter, were grown overnight at 30°C and inoculated at low density into medium containing 20% BCS at 37°C. Cells were harvested 3 h postinduction and stained with 0.2 μg/ml Calcofluor White for 20 min. Each sample was washed in phosphate-buffered saline, and a DIC image to detect cell morphology (A–C) and fluorescence images to detect GFP (D–F) and chitin (G–I) were captured using a fluorescence microscope with appropriate filters. Bar, 10 μm.
cdc11Δ Cells Display Reduced Filamentation in Agar
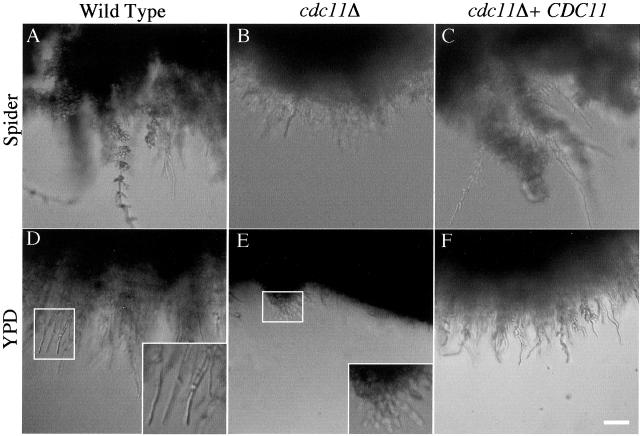
The septin deletion strains were also tested for invasive growth when embedded in agar. The cdc10Δ strain appeared to be similar to wild type. In contrast, the cdc11Δ mutant showed reduced invasive growth in all media tested. cdc11Δ colonies tended to have a solid disk-like appearance in agar, whereas other strains appeared as fuzzy orbs radiating out in all directions. This difference was not due to a general growth defect but instead reflected a reduced ability to penetrate the agar. The defect was most pronounced in YPD medium, where microscopic analysis showed that there were fewer and shorter extensions of growth into the agar radiating out from cdc11Δ colonies (Figure 9E, inset). Colonies of other strains embedded in YPD displayed longer invasions of growth that were made up primarily of elongated cells or chains of oval cells (Figure 9D, inset). In Spider medium, wild-type and cdc10Δ colonies formed long extensions of filamentous growth that appeared thick due to lateral budding in the form of both elongated and round budded cells (Figure 9A). The cdc11Δ strain was completely lacking in the thick invasive branches, instead displaying only a limited amount of what seemed to be pseudohyphal growth (Figure 9B). This trend was also observed in N-acetyl-d-glucosamine and 4% BCS (our unpublished data). Integration of a single copy of CDC11 reverted the invasion defects (Figure 9, C and F). Thus, Cdc11p seems to be important for the ability to carry out invasive growth.
Figure 9.
cdc11Δ is defective for filamentation in agar medium. Wild-type strain BWP17 (A and D), cdc11Δ strain YAW9 (B and E), and strain YAW10 in which cdc11Δ is complemented by an integrated copy of wild-type CDC11 (C and F) were grown overnight at 30°C. Approximately100 cells for each strain were embedded into 40 ml of molten agar medium of the indicated type and plated. Plates were incubated for four days at 37°C and edges of individual colonies fully embedded in agar were photographed at low magnification. Insets in D and E show an enlargement of the boxed area. Bar, 40 μm.
DISCUSSION
Originally identified for their role in cytokinesis in S. cerevisiae, septins have since been found to play a broader role in other morphogenetic events in yeast, flies, and mammals. In this study, we analyzed the roles of septins in the pathogenic yeast C. albicans, which has the capacity to grow with a variety of morphologies. The ability of C. albicans to switch between diverse morphologies is regulated, in part, by interaction with host cells and is thought to be one of the most important factors governing its fitness as a pathogen. Analysis of the C. albicans genome sequence identified seven open reading frames with strong similarity to the septin protein family. The four septins encoded by CDC3, CDC10, CDC11, and CDC12 assembled into a ring at the future site of septation during budding, pseudohyphal, and hyphal growth of C. albicans. Interestingly, the homologs of the sporulation-specific septins of S. cerevisiae (Spr3 and Spr28) displayed the least similarity to their counterparts (Figure 1). This greater degree of divergence correlates with the failure to detect sporulation of C. albicans. It has been proposed, based on comparisons with genes involved in the sexual cycle of S. cerevisiae, that C. albicans may undergo meiosis in an alternative manner (Tzung et al., 2001).
Cdc10p and Cdc11p Display Distinct Functional Properties during C. albicans Budding
Analysis of the septin mutants identified interesting differences between C. albicans and S. cerevisiae. Whereas a S. cerevisiae cdc10Δ strain is reported to show an abnormal morphology at any temperature, the majority of the C. albicans cdc10Δ cells appeared to be wild type at 30°C or below, but were defective at elevated temperature. At 42°C, these cells were not able to localize other septins to the bud neck, probably contributing to the more severe phenotype observed at this temperature. S. cerevisiae septin mutants at elevated temperature display a similar elongated bud morphology and fail to maintain localization of other septins at the bud neck, but they also do not remain viable (Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991; Frazier et al., 1998; Robinson et al., 1999). Surprisingly, deletion of CDC10 in C. albicans does not strongly affect growth rate or viability even at 42°C, indicating that cytokinesis must occur at some, albeit lower, frequency. Thus, C. albicans differs from S. cerevisiae in that the cells can continue to proliferate successfully in the absence of detectable septins rings.
C. albicans cdc10Δ and cdc11Δ mutants were similar in that both displayed defects in cytokinesis and aberrant chitin localization. However, they differed in that cdc11Δ cells displayed a high degree of budding from a site adjacent to the previous bud (axial budding), whereas cdc10Δ cells primarily formed long chain of cells connected end to end (polar budding). Additionally, cdc11Δ cells formed fewer of the extensively elongated buds at 42°C that were commonly seen for cdc10Δ cells. The difference between these mutants may relate to the fact that cdc11Δ cells retained localization of other septins to a ring at the bud neck at 42°C. On the other hand, the difference could also be due to a differential ability of the Cdc10p and Cdc11p septins to interact with other proteins that are recruited to the bud neck, a possibility that is consistent with genetic evidence for some septin-binding proteins in S. cerevisiae (Barral et al., 1999; Okuzaki and Nojima, 2001).
Septins Influence C. albicans Hyphal Morphogenesis
Septin localization in germ tubes was distinct from that observed during budding or pseudohyphal growth. Instead of forming a well defined ring, the septins localized in a diffuse array at the plasma membrane domain where germ tube growth was initiated. This array was more difficult to detect than the ring, which may account for discrepancies in previous reports (Gale et al., 2001; Sudbery, 2001). Analysis of septin gene expression suggests that there is not a hyphal-specific septin gene (DiDomenico et al., 1994; our unpublished data). Thus, this change in septin localization is apparently due to changes in septin regulation during hyphal growth. The distinction between these different patterns of septin localization allow hyphal and pseudohphal growth to be distinguished at the earliest stages, before cell morphology can be assessed, or later when the overall morphologies can be quite similar. At later stages, when the first septum began to form within the germ tube, the septins were observed to form a tight ring structure similar to that observed in budding or pseudohyphal cells. Interestingly, the septin rings formed during hyphal growth did not appear to disassemble after septation as rapidly as in budding cells, and often the ring on the daughter side disappeared more quickly than that on the mother cell side. This difference may reflect a delay in cell cycle progression of the older cells near the base of the hypha. Mother cells have been reported to swell their vacuoles to contribute their cytoplasmic constituents to assist the more rapid tip growth and may therefore need more time to initiate a new division cycle (Gow and Gooday, 1984).
Deletion analysis demonstrated that the full set of septins is not vital to hyphal morphogenesis in that the cdc10Δ and cdc11Δ mutants were not grossly defective in serum-induced hyphal growth, although some defects were apparent. The hyphae of the mutant cells typically grow with a greater degree of curvature, which may reflect a defect in cell wall construction, as suggested also by flaws in septum formation and a nonhomogenous distribution of cell wall chitin. In addition, the mutants differed from wild type in selection of the sites for secondary germ tube emergence. In contrast to the seemingly random selection of sites in wild-type cells, the mutants often selected sites that were directly adjacent to the previous hypha. This was particularly evident for the cdc11Δ strain, which also showed a bias toward an axial budding pattern, suggesting that the selection of sites for buds and germ tubes both involve septin-dependent mechanisms. The cdc11Δ cells were also defective in undergoing filamentous growth when embedded in agar.
Formation of Small Germ Tube-like Protuberances
Time-lapse studies showed that serum treatment caused wild-type cells to form a series of short, germ-tube like protuberances at seemingly random locations before extending one protuberance into full hyphal growth. The septins seem to play a role in this process, because the cdc10Δ and cdc11Δ mutants displayed a lower frequency of this switching behavior. A similar type of small protuberances may have been observed for a special set of conditions involving induction with N-acetyl-d-glucosamine (Hubbard et al., 1985). Although to our knowledge this behavior has not been reported previously for C. albicans cells induced with serum, this effect does not seem to be limited to the time-lapse studies. Small protuberances can be observed at sites distinct from the main germ tube both in some of the previously published micrographs of serum-induced cells (Herrero et al., 1999) and in cells from liquid cultures that we have analyzed (our unpublished data). Several factors suggest that this switching phenomenon may play a role in Candida pathogenesis. In particular, these structures could play a role analogous to that of the primary germ tube of certain plant pathogens, such as Blumeria graminis. The conidia of B. graminis initially form a small primary germ tube to help adhere to the plant epidermal layer and then form an elongated secondary germ tube that invades deeper into the plant tissue (Heitefuss, 2001). Because C. albicans germ tubes are reported to display increased adherence to mammalian cells (Hostetter, 1996; Calderone, 1998; Gale et al., 1998; Staab et al., 1999), these short protuberances could play an important role in mediating the adherence to host cells during an infection.
Mechanisms of Septin Action
Studies on the role of the septin ring during budding have led to the model that the septins act as a scaffold to recruit proteins to the neck region to ensure proper bud morphogenesis and cytokinesis. Additional studies indicate that the septins can also function as a boundary that helps to maintain polarized growth in the bud by restricting actin and other proteins from the mother cell (Barral et al., 2000; Takizawa et al., 2000). Studies on mammalian cells have further indicated that septins can contribute to regulated secretion (Kartmann and Roth, 2001). These mechanisms for septin action are not mutually exclusive and may all contribute to bud morphogenesis and cytokinesis. In addition, these models for septin action are consistent with what was observed in the analysis of septin mutants in C. albicans. For example, septins localize to the sites of germ tube formation and may act to promote proper hyphal morphogenesis by recruiting proteins such as Int1p, which has been shown to bind to septins and is necessary for hyphal morphogenesis under some conditions (Gale et al., 1998, 2001). Septins may also function to define the shape of the hyphae by acting as a boundary domain that helps to restrict actin to the growing tip. Cdc10-GFP was often observed at the leading edge of germ tubes (Figure 2), and a failure to fine-tune the site of actin polarization could account for the greater degree of curvature in hyphae formed by septin mutants (Figure 7). In addition, because morphogenesis in yeast is primarily due to deposition of secretory vesicles at specific sites in the plasma membrane, septins could play a direct role in directing early tip morphogenesis by differentially regulating secretion during bud and hyphal emergence.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Dana Davis (University of Minnesota, St. Paul, MN) and Aaron Mitchell (Columbia University) for strains, plasmids, and invaluable advice, Drs. Aaron Neiman and Neta Dean (SUNY Stony Brook) for useful suggestions, and Dr. Alistair Brown (University of Aberdeen, Aberdeen, Scotland) and Stephen Martin (SUNY Stony Brook) for plasmids. This work was supported by grant R01 AI47837 from the National Institutes of Health (to J.B.K.). Digital camera equipment was obtained through a Targeted Research Opportunity Grant from the Stony Brook School of Medicine.
Abbreviations used:
- BCS
bovine calf serum
- DIC
differential interference contrast
- GFP
green fluorescent protein
- YPD
yeast peptone dextrose media
Footnotes
Online version of this article contains supplemental tables. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0013. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0013.
REFERENCES
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DA, Feldmann PJ, Bovey M, Gow NA, Brown AJ. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–5360. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Buffo J, Herman MA, Soll DR. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984;85:21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976a;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B, Goetsch L. Loss of the filamentous ring in cytokinesis-defective mutants of budding yeast. J Cell Biol. 1976b;70:35a. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. The INT1 of Candida albicans. Trends Microbiol. 1998;6:300–303. doi: 10.1016/s0966-842x(98)01321-3. [DOI] [PubMed] [Google Scholar]
- Carroll CW, Altman R, Schieltz D, Yates JR, Kellogg D. The septins are required for the mitosis-specific activation of the Gin4 kinase. J Cell Biol. 1998;143:709–717. doi: 10.1083/jcb.143.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Mischke M, Mitchell E, Herskowitz I, Pringle JR. Role of Bud3p in producing the axial budding pattern of yeast. J Cell Biol. 1995;129:767–778. doi: 10.1083/jcb.129.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Kiehart DP. Septins may form a ubiquitous family of cytoskeletal filaments. J Cell Biol. 1996;134:1345–1348. doi: 10.1083/jcb.134.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack BP, Bertram G, Egerton M, Gow NA, Falkow S, Brown AJ. Yeast-enhanced green fluorescent protein (yEGFP) a reporter of gene expression in Candida albicans. Microbiology. 1997;143:303–311. doi: 10.1099/00221287-143-2-303. [DOI] [PubMed] [Google Scholar]
- De Virgilio C, DeMarini DJ, Pringle JR. SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells. Microbiology. 1996;142:2897–2905. doi: 10.1099/13500872-142-10-2897. [DOI] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AE, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico BJ, Brown NH, Lupisella J, Greene JR, Yanko M, Koltin Y. Homologs of the yeast neck filament associated genes: isolation and sequence analysis of Candida albicans CDC3 and CDC10. Mol Gen Genet. 1994;242:689–698. doi: 10.1007/BF00283424. [DOI] [PubMed] [Google Scholar]
- Enloe B, Diamond A, Mitchell AP. A single-transformation gene function test in diploid Candida albicans. J Bacteriol. 2000;182:5730–5736. doi: 10.1128/jb.182.20.5730-5736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H, Goetsch L, Pringle JR. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J Cell Biol. 1996;132:399–411. doi: 10.1083/jcb.132.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H, Peifer M, Pringle JR. Localization and possible functions of Drosophila septins. Mol Biol Cell. 1995;6:1843–1859. doi: 10.1091/mbc.6.12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CM, Kellogg D. Septins: cytoskeletal polymers or signaling GTPases? Trends Cell Biol. 1999;9:387–394. doi: 10.1016/s0962-8924(99)01632-3. [DOI] [PubMed] [Google Scholar]
- Flescher EG, Madden K, Snyder M. Components required for cytokinesis are important for bud site selection in yeast. J Cell Biol. 1993;122:373–386. doi: 10.1083/jcb.122.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SK, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev Genet. 1991;12:281–292. doi: 10.1002/dvg.1020120405. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Wong ML, Longtine MS, Pringle JR, Mann M, Mitchison TJ, Field C. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol. 1998;143:737–749. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C, Gerami-Nejad M, McClellan M, Vandoninck S, Longtine MS, Berman J. Candida albicans Int1p Interacts with the septin ring in yeast and hyphal cells. Mol Biol Cell. 2001;12:3538–3549. doi: 10.1091/mbc.12.11.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CA, Bendel CM, McClellan M, Hauser M, Becker JM, Berman J, Hostetter MK. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- Gerami-Nejad M, Berman J, Gale CA. Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast. 2001;18:859–864. doi: 10.1002/yea.738. [DOI] [PubMed] [Google Scholar]
- Giot L, Konopka JB. Functional analysis of the interaction between Afr1p and the Cdc12p septin, two proteins involved in pheromone-induced morphogenesis. Mol Biol Cell. 1997;8:987–998. doi: 10.1091/mbc.8.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Gow NA, Gooday GW. A model for the germ tube formation and mycelial growth form of Candida albicans. Sabouraudia. 1984;22:137–144. doi: 10.1080/00362178485380211. [DOI] [PubMed] [Google Scholar]
- Haarer BK, Pringle JR. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10-nm filaments in the mother-bud neck. Mol Cell Biol. 1987;7:3678–3687. doi: 10.1128/mcb.7.10.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hazan I, Sepulveda-Becerra M, Liu H. Hyphal elongation Is regulated independently of cell cycle in Candida albicans. Mol Biol Cell. 2002;13:134–145. doi: 10.1091/mbc.01-03-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitefuss R. Defense reactions of plants to fungal pathogens: principles and perspectives, using powdery mildew on cereals as an example. Naturwissenschaften. 2001;88:273–283. doi: 10.1007/s001140100226. [DOI] [PubMed] [Google Scholar]
- Herrero AB, Lopez MC, Fernandez-Lago L, Dominguez A. Candida albicans and Yarrowia lipolytica as alternative models for analyzing budding patterns and germ tube formation in dimorphic fungi. Microbiology. 1999;145:2727–2737. doi: 10.1099/00221287-145-10-2727. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hostetter MK. Adhesion and morphogenesis in Candida albicans. Pediatr Res. 1996;39:569–573. doi: 10.1203/00006450-199604000-00001. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Hubbard MJ, Sullivan PA, Shepherd MG. Morphological studies of N-acetylglucosamine induced germ tube formation by Candida albicans. Can J Microbiol. 1985;31:696–701. doi: 10.1139/m85-132. [DOI] [PubMed] [Google Scholar]
- Kartmann B, Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci. 2001;114:839–844. doi: 10.1242/jcs.114.5.839. [DOI] [PubMed] [Google Scholar]
- Kim HB, Haarer BK, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Fares H, Pringle JR. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J Cell Biol. 1998;143:719–736. doi: 10.1083/jcb.143.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino A, Tanaka K, Kamei T, Umikawa M, Fujiwara T, Takai Y. Shs1p: a novel member of septin that interacts with Spa2p, involved in polarized growth in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1998;251:732–736. doi: 10.1006/bbrc.1998.9541. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokinesis and morphogenesis but have no essential function in embryogenesis. J Cell Sci. 2000;113:3825–3837. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- Odds FC. Candida and Candidosis. Philadelphia: Bailliere Tindall; 1988. [Google Scholar]
- Okuzaki D, Nojima H. Kcc4 associates with septin proteins of Saccharomyces cerevisiae. FEBS Lett. 2001;489:197–201. doi: 10.1016/s0014-5793(01)02104-4. [DOI] [PubMed] [Google Scholar]
- Ozsarac N, Bhattacharyya M, Dawes IW, Clancy MJ. The SPR3 gene encodes a sporulation-specific homologue of the yeast CDC3/10/11/12 family of bud neck microfilaments and is regulated by ABFI. Gene. 1995;164:157–162. doi: 10.1016/0378-1119(95)00438-c. [DOI] [PubMed] [Google Scholar]
- Robinson LC, Bradley C, Bryan JD, Jerome A, Kweon Y, Panek HR. The Yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Mol Biol Cell. 1999;10:1077–1092. doi: 10.1091/mbc.10.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Slater ML, Bowers B, Cabib E. Formation of septum-like structures at locations remote from the budding sites in cytokinesis-defective mutants of Saccharomyces cerevisiae. J Bacteriol. 1985;162:763–767. doi: 10.1128/jb.162.2.763-767.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll DR, Mitchell LH. Filament ring formation in the dimorphic yeast Candida albicans. J Cell Biol. 1983;96:486–493. doi: 10.1083/jcb.96.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- Sudbery PE. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol Microbiol. 2001;41:19–31. doi: 10.1046/j.1365-2958.2001.02459.x. [DOI] [PubMed] [Google Scholar]
- Tachikawa H, Bloecher A, Tatchell K, Neiman AM. A Gip1p-Glc7p phosphatase complex regulates septin organization and spore wall formation. J Cell Biol. 2001;155:797–808. doi: 10.1083/jcb.200107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Trimble WS. Septins: a highly conserved family of membrane-associated GTPases with functions in cell division and beyond. J Membr Biol. 1999;169:75–81. doi: 10.1007/s002329900519. [DOI] [PubMed] [Google Scholar]
- Tzung KW, et al. Genomic evidence for a complete sexual cycle in Candida albicans. Proc Natl Acad Sci USA. 2001;98:3249–3253. doi: 10.1073/pnas.061628798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.