Abstract
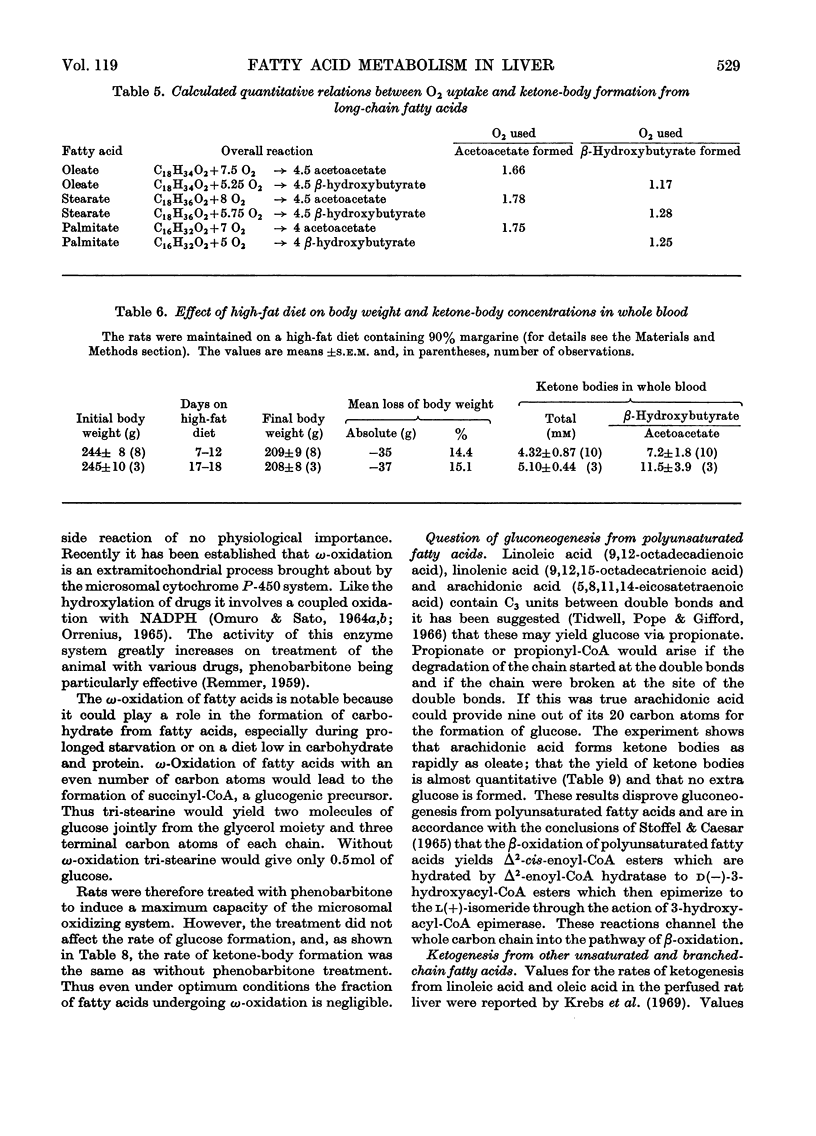
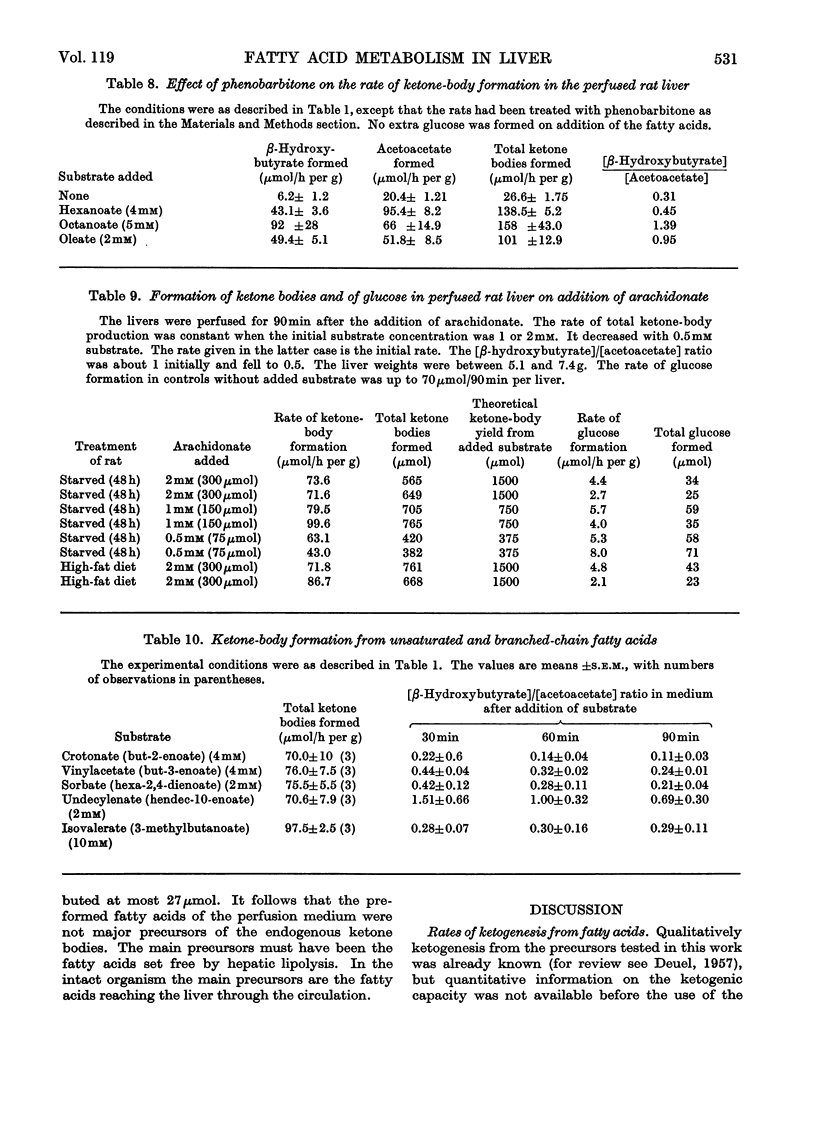
1. The formation of acetoacetate, β-hydroxybutyrate and glucose was measured in the isolated perfused rat liver after addition of fatty acids. 2. The rates of ketone-body formation from ten fatty acids were approximately equal and independent of chain length (90–132μmol/h per g), with the exception of pentanoate, which reacted at one-third of this rate. The [β-hydroxybutyrate]/[acetoacetate] ratio in the perfusion medium was increased by long-chain fatty acids. 3. Glucose was formed from all odd-numbered fatty acids tested. 4. The rate of ketone-body formation in the livers of rats kept on a high-fat diet was up to 50% higher than in the livers of rats starved for 48h. In the livers of fat-fed rats almost all the O2 consumed was accounted for by the formation of ketone bodies. 5. The ketone-body concentration in the blood of fat-fed rats rose to 4–5mm and the [β-hydroxybutyrate]/[acetoacetate] ratio rose to 11.5. 6. When the activity of the microsomal mixed-function oxidase system, which can bring about ω-oxidation of fatty acids, was induced by treatment of the rat with phenobarbitone, there was no change in the ketone-body production from fatty acids, nor was there a production of glucose from even-numbered fatty acids. The latter would be expected if ω-oxidation occurred. Thus ω-oxidation did not play a significant role in the metabolism of fatty acids. 7. Arachidonate was almost quantitatively converted into ketone bodies and yielded no glucose, demonstrating that gluconeogenesis from poly-unsaturated fatty acids with an even number of carbon atoms does not occur. 8. The rates of ketogenesis from unsaturated fatty acids (sorbate, undecylenate, crotonate, vinylacetate) were similar to those from the corresponding saturated fatty acids. 9. Addition of oleate together with shorter-chain fatty acids gave only a slightly higher rate of ketone-body formation than oleate alone. 10. Glucose, lactate, fructose, glycerol and other known antiketogenic substances strongly inhibited endogenous ketogenesis but had no effects on the rate of ketone-body formation in the presence of 2mm-oleate. Thus the concentrations of free fatty acids and of other oxidizable substances in the liver are key factors determining the rate of ketogenesis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill G. J., Jr, Owen O. E., Morgan A. P. The consumption of fuels during prolonged starvation. Adv Enzyme Regul. 1968;6:143–150. doi: 10.1016/0065-2571(68)90011-3. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Das M. L., Orrenius S., Ernster L. On the fatty acid and hydrocarbon hydroxylation in rat liver microsomes. Eur J Biochem. 1968 May;4(4):519–523. doi: 10.1111/j.1432-1033.1968.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Edson N. L. Ketogenesis-antiketogenesis: Substrate competition in liver. Biochem J. 1936 Oct;30(10):1862–1869. doi: 10.1042/bj0301862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. W. Studies in the ketosis of fasting. J Clin Invest. 1967 Aug;46(8):1283–1296. doi: 10.1172/JCI105621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- KREBS H. A. The physiological role of the ketone bodies. Biochem J. 1961 Aug;80:225–233. doi: 10.1042/bj0800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoff R. K., Kipnis D. M. Studies of the metabolic effects of acute insulin deficiency. I. Mechanism of impairment of hepatic fatty acid and protein synthesis. Diabetes. 1966 Jul;15(7):443–450. doi: 10.2337/diab.15.7.443. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Rate control of the tricarboxylic acid cycle. Adv Enzyme Regul. 1970;8:335–353. doi: 10.1016/0065-2571(70)90028-2. [DOI] [PubMed] [Google Scholar]
- Leloir L. F., Muñoz J. M. Fatty acid oxidation in liver. Biochem J. 1939 May;33(5):734–746. doi: 10.1042/bj0330734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMMER H. [The acceleration of evipan oxidation and the demethylation of methylaminopyrine by barbiturates]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1959;237:296–307. [PubMed] [Google Scholar]
- ROSSI C. R., GIBSON D. M. ACTIVATION OF FATTY ACIDS BY A GUANOSINE TRIPHOSPHATE-SPECIFIC THIOKINASE FROM LIVER MITOCHONDRIA. J Biol Chem. 1964 Jun;239:1694–1699. [PubMed] [Google Scholar]
- SMITH R. M., OSBORNE-WHITE W. S. METABOLISM OF PROPIONATE BY SHEEP LIVER. OXIDATION OF PROPIONATE BY HOMOGENATES. Biochem J. 1965 May;95:411–422. doi: 10.1042/bj0950411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadie W. C. FAT METABOLISM IN DIABETES MELLITUS. J Clin Invest. 1940 Nov;19(6):843–861. doi: 10.1172/JCI101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Verkade P. E., Van Der Lee J. Researches on fat metabolism. II. Biochem J. 1934;28(1):31–40. doi: 10.1042/bj0280031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Krebs H. A. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem J. 1968 Jul;108(3):353–361. doi: 10.1042/bj1080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Browning E. T., Scholz R. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4607–4616. [PubMed] [Google Scholar]


