Abstract
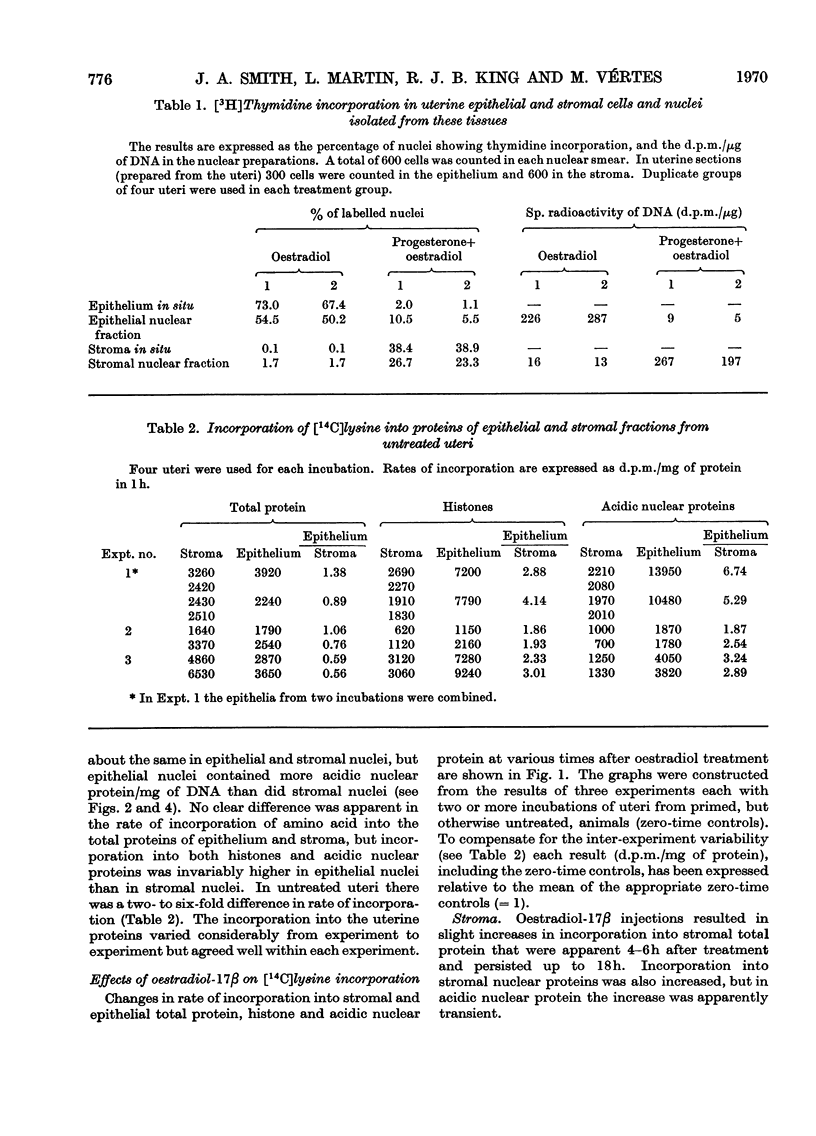
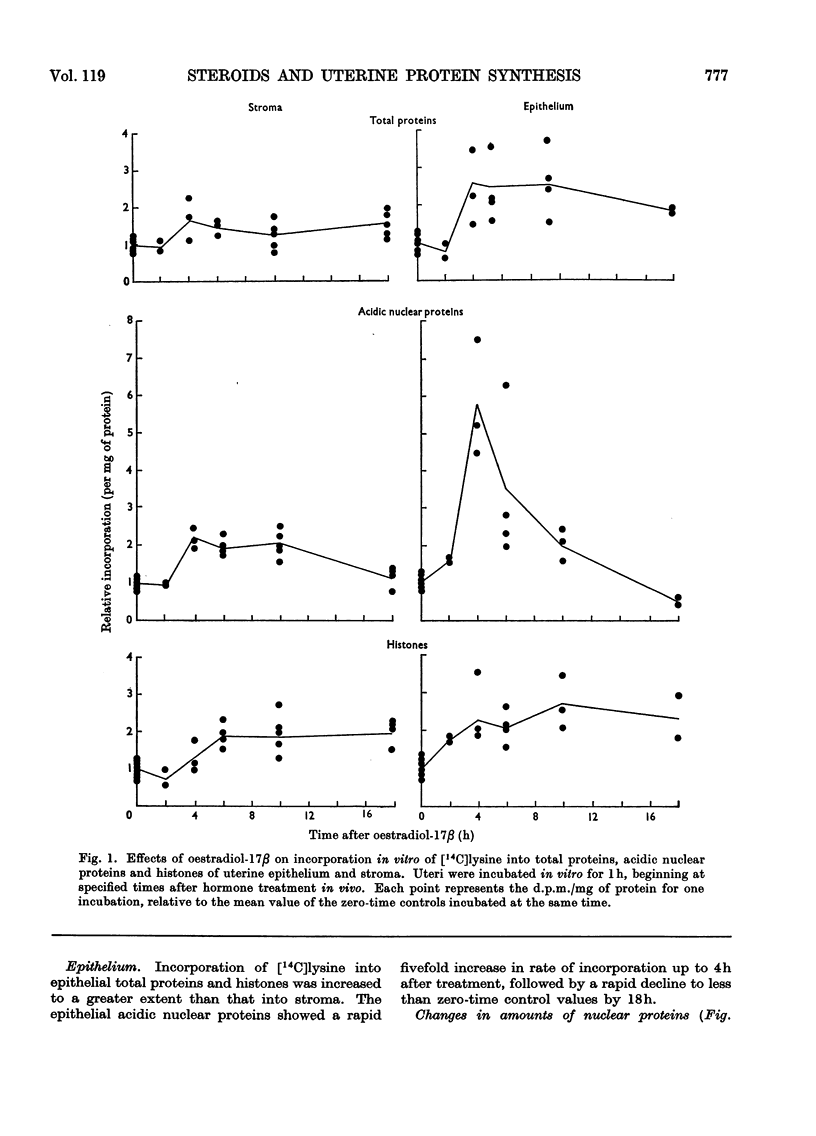
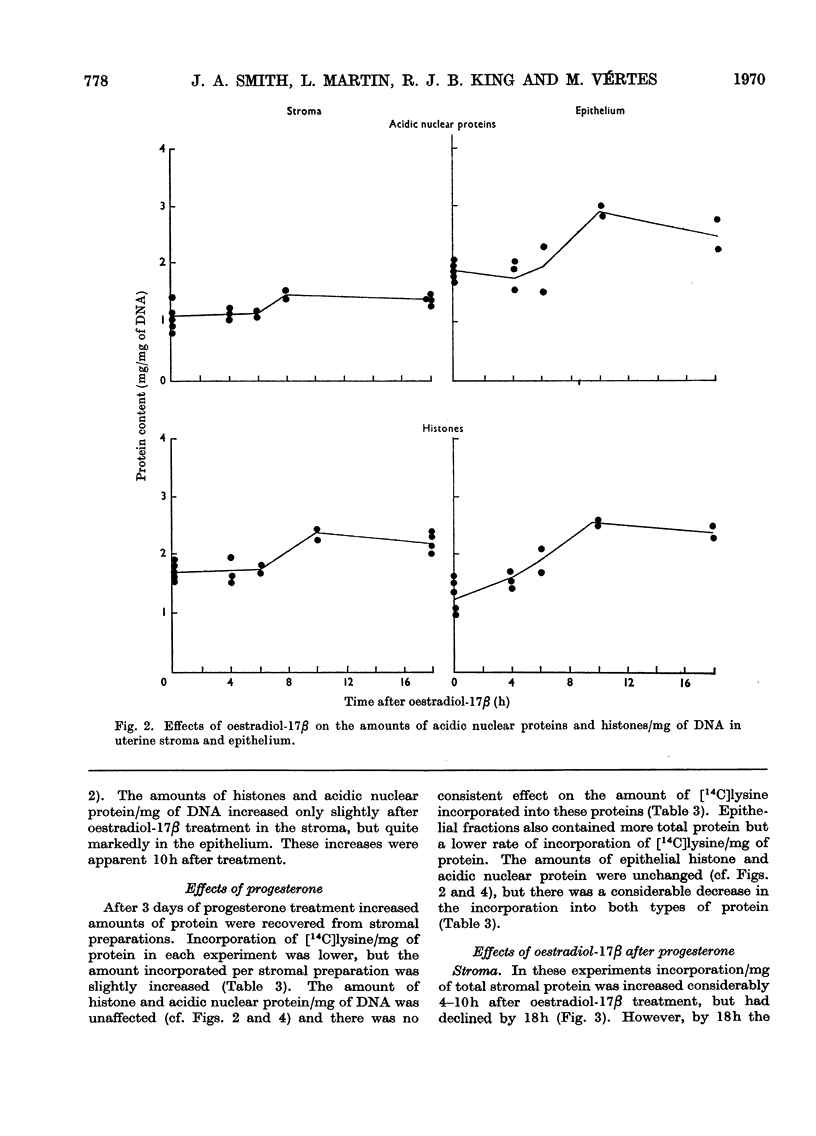
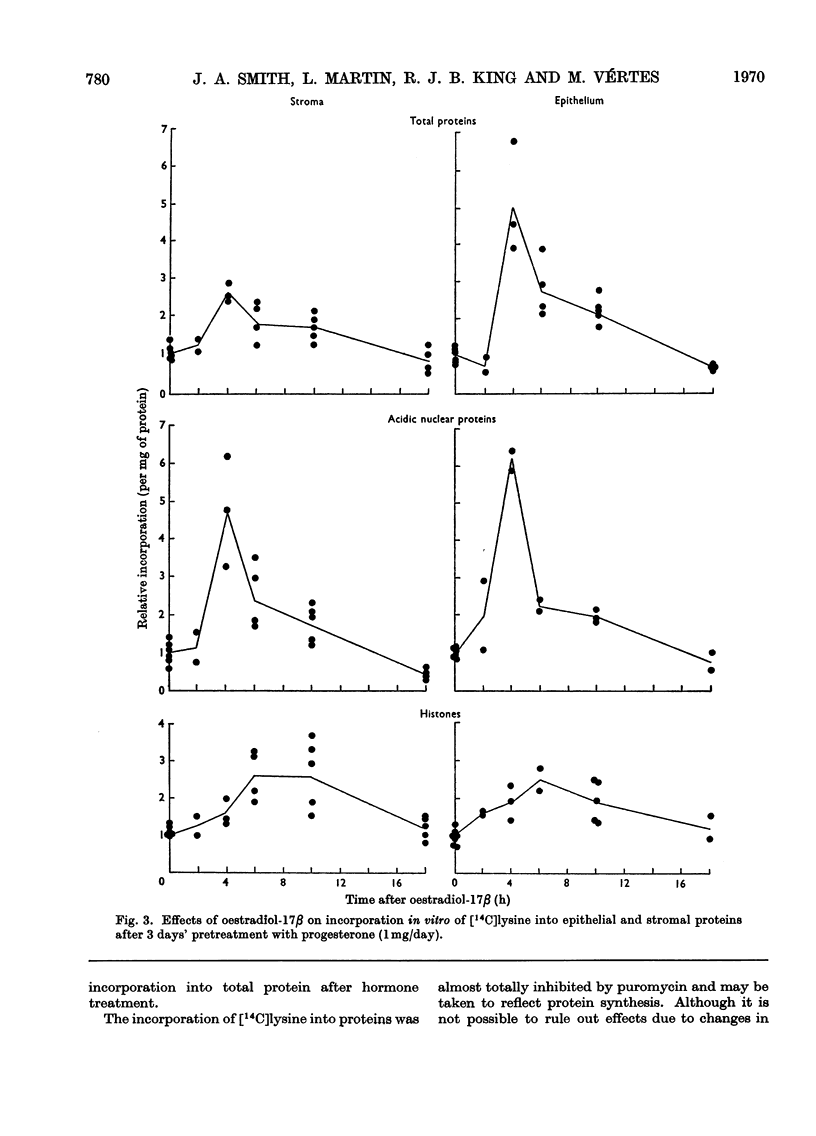
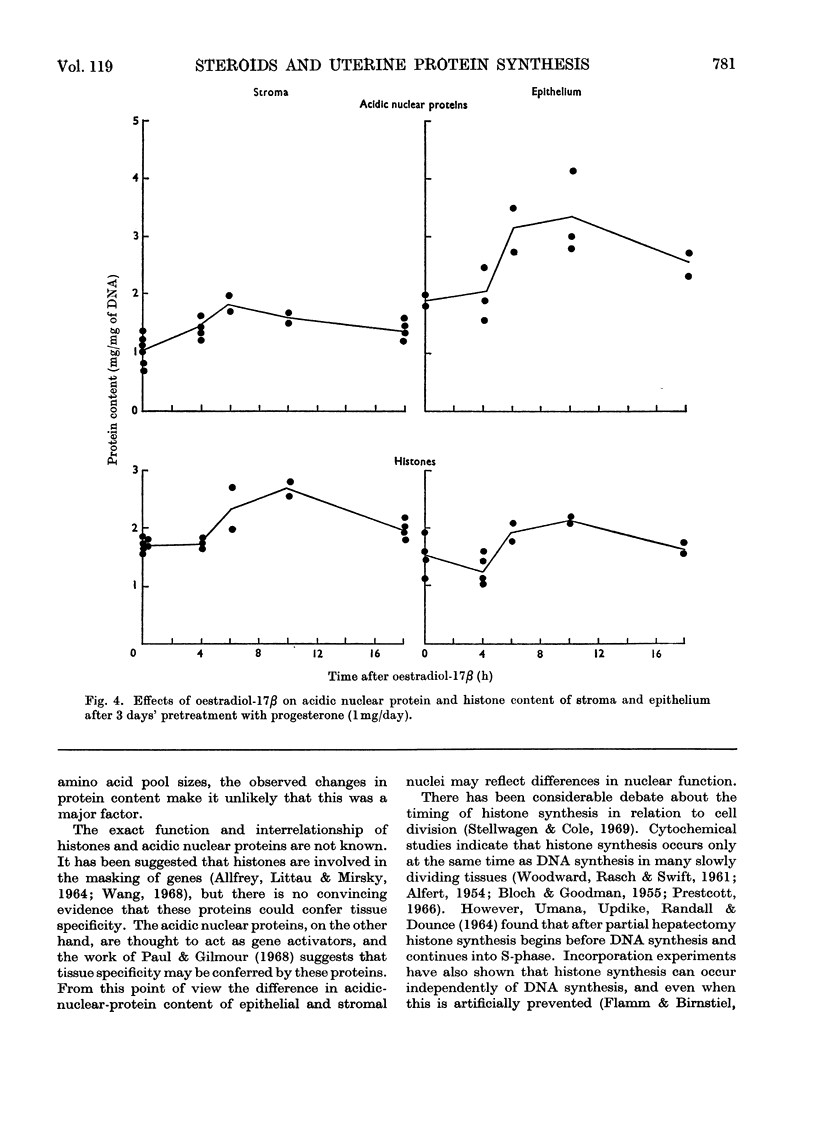
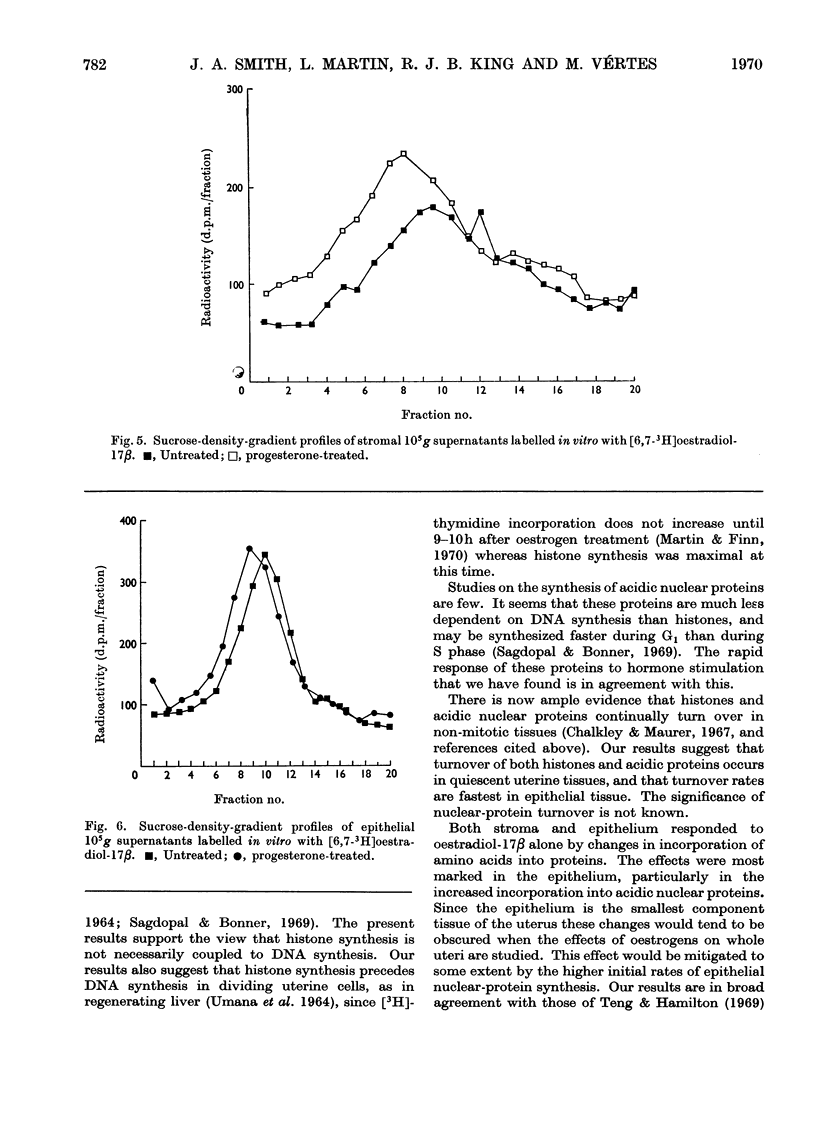
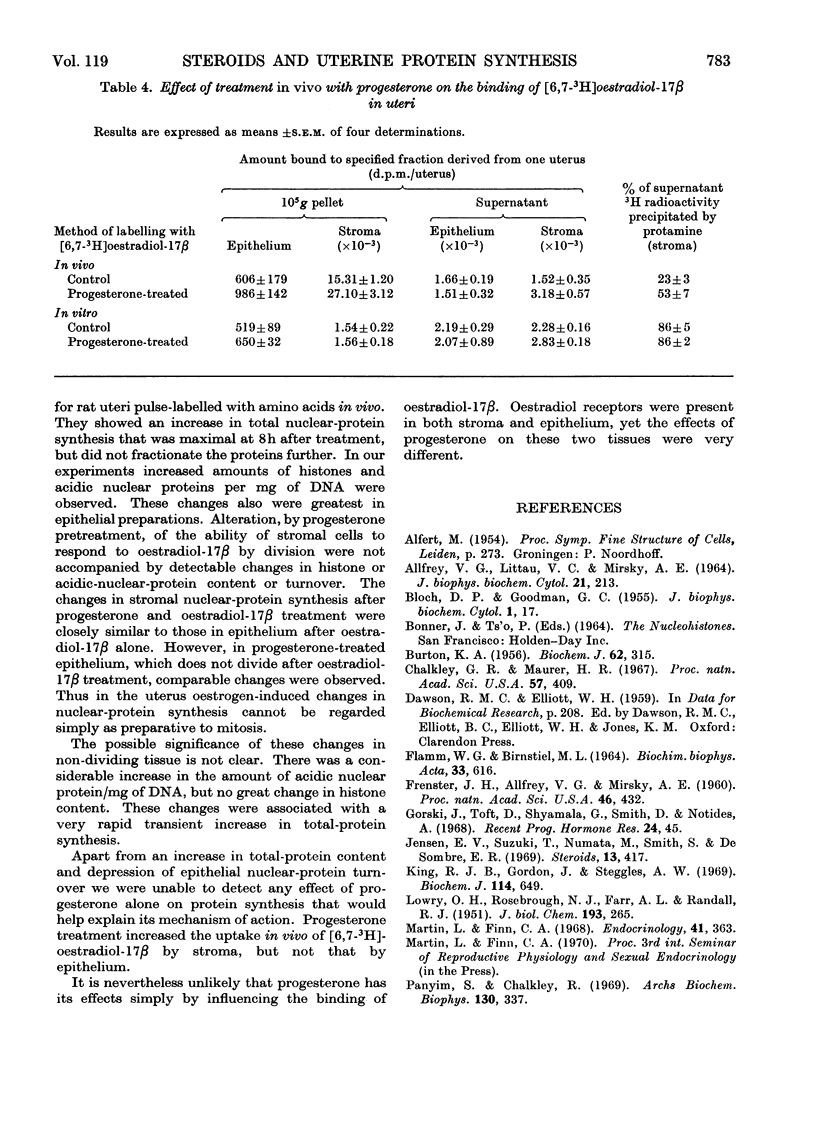
1. A method is described for separating uterine epithelium that is 80% pure and connective-tissue stroma that is 60% pure. This was used to study the effects of steroid hormones on total and nuclear-protein synthesis in these tissues. 2. Oestradiol-17β given alone produces mitoses in the epithelium but not in the stroma. It stimulated incorporation in vitro of [14C]lysine into total protein, histones and acidic nuclear proteins to a greater extent in epithelium than stroma. Incorporation into acidic nuclear proteins was most markedly stimulated, reaching four to six times the normal value 4h after treatment, and then declining rapidly. This peak was only seen in epithelial preparations. 3. After pretreatment with progesterone, oestradiol-17β has the reverse effect, producing mitoses only in stroma. Progesterone alone had no effect on the amounts or rates of incorporation of [14C]lysine into stromal nuclear proteins, but changes after oestradiol-17β treatment were similar to those seen in epithelium with oestradiol-17β alone. In the epithelium, progesterone alone depressed incorporation into histones and acidic nuclear proteins, but did not abolish the subsequent response to oestradiol-17β. With this treatment there was a rapid, large and transient increase in incorporation into epithelial total protein not seen with oestradiol-17β alone. 4. Progesterone had no qualitative effect on the distribution of specific oestrogen-binding proteins, as judged by sucrose-density-gradient centrifugation. However, progesterone treatment increased the uptake in vivo of [6,7-3H]oestradiol-17β by stroma, and it is possible that this is important although the differences were not apparent after labelling in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., LITTAU V. C., MIRSKY A. E. METHODS FOR THE PURIFICATION OF THYMUS NUCLEI AND THEIR APPLICATION TO STUDIES OF NUCLEAR PROTEIN SYNTHESIS. J Cell Biol. 1964 May;21:213–231. doi: 10.1083/jcb.21.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH D. P., GODMAN G. C. A microphotometric study of the syntheses of desoxyribonucleic acid and nuclear histone. J Biophys Biochem Cytol. 1955 Jan;1(1):17–28. doi: 10.1083/jcb.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAMM W. G., BIRNSTIEL M. L. INHIBITION OF DNA REPLICATION AND ITS EFFECT ON HISTONE SYNTHESIS. Exp Cell Res. 1964 Feb;33:616–619. doi: 10.1016/0014-4827(64)90035-7. [DOI] [PubMed] [Google Scholar]
- Frenster J. H., Allfrey V. G., Mirsky A. E. METABOLISM AND MORPHOLOGY OF RIBONUCLEOPROTEIN PARTICLES FROM THE CELL NUCLEUS OF LYMPHOCYTES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):432–444. doi: 10.1073/pnas.46.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Numata M., Smith S., DeSombre E. R. Estrogen-binding substances of target tissues. Steroids. 1969 Apr;13(4):417–427. doi: 10.1016/0039-128x(69)90053-1. [DOI] [PubMed] [Google Scholar]
- King R. J., Gordon J., Steggles A. W. The properties of a nuclear acidic protein fraction that binds [6,7-3H]oestradiol-17beta. Biochem J. 1969 Sep;114(3):649–657. doi: 10.1042/bj1140649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin L., Finn C. A. Hormonal regulation of cell division in epithelial and connective tissues of the mouse uterus. J Endocrinol. 1968 Jul;41(3):363–371. doi: 10.1677/joe.0.0410363. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The syntheses of total macronuclear protein, histone, and DNA during the cell cycle in Euplotes eurystomus. J Cell Biol. 1966 Oct;31(1):1–9. doi: 10.1083/jcb.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadgopal A., Bonner J. The relationship between histone and DNA synthesis in HeLa cells. Biochim Biophys Acta. 1969 Aug 20;186(2):349–357. doi: 10.1016/0005-2787(69)90013-6. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., King R. J. The use of protamine to study [6,7-3H] oestradiol-17-beta binding in rat uterus. Biochem J. 1970 Aug;118(5):695–701. doi: 10.1042/bj1180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. Role of chromatin in estrogen action in the uterus. II. Hormone-induced synthesis of nonhistone acidic proteins which restore histone-inhibited DNA-dependent RNA synthesis. Proc Natl Acad Sci U S A. 1969 Jun;63(2):465–472. doi: 10.1073/pnas.63.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODARD J., RASCH E., SWIFT H. Nucleic acid and protein metabolism during the mitotic cycle in Vicia faba. J Biophys Biochem Cytol. 1961 Feb;9:445–462. doi: 10.1083/jcb.9.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]



