Abstract
Glucagon (GCG) like peptide 1 (GLP-1) has emerged as a powerful player in regulating metabolism and a promising therapeutic target for various chronic diseases. This review delves into the physiological roles of GLP-1, exploring its impact on glucose homeostasis, insulin secretion, and satiety. We examine the compelling evidence supporting GLP-1 receptor agonists (GLP-1RAs) in managing type 2 diabetes (T2D), obesity, and other diseases. The intricate molecular mechanisms underlying GLP-1RAs are explored, including their interactions with pathways like extracellular signal-regulated kinase 1/2 (ERK1/2), activated protein kinase (AMPK), cyclic adenine monophosphate (cAMP), mitogen-activated protein kinase (MAPK), and protein kinase C (PKC). Expanding our understanding, the review investigates the potential role of GLP-1 in cancers. Also, microribonucleic acid (RNA) (miRNAs), critical regulators of gene expression, are introduced as potential modulators of GLP-1 signaling. We delve into the link between miRNAs and T2D obesity and explore specific miRNA examples influencing GLP-1R function. Finally, the review explores the rationale for seeking alternatives to GLP-1RAs and highlights natural products with promising GLP-1 modulatory effects.
Keywords: GLP-1, GLP-1RAs, Diabetes, Obesity, Cancer, miRNAs, Natural products
Highlights
-
•
A broad therapeutic value of GLP-1RAs, including their benefits in glycemic control, weight management, and cardiovascular health.
-
•
Understanding GLP-1’s roles potentially prevent and manage obesity, T2D, and some forms of cancer.
-
•
Natural products identified with GLP-1 modulating properties provide a basis for alternative therapies to injectable GLP-1RAs.
-
•
This review integrates recent advances on GLP-1's physiological and therapeutic roles, highlighting miRNA involvement.
-
•
Novel regulatory mechanisms by GLP-1 and miRNAs could be harnessed for future therapeutic strategies, particularly in complex diseases.
1. Introduction
A hormone released by intestinal L cells in response to nutrients, GLP-1, plays a complex and diverse role in regulating metabolism [3]. This review explores GLP-1's physiological functions, therapeutic potential, and intricate molecular mechanisms. It also discusses the benefits of natural compounds that modulate GLP-1 and highlights the importance of finding alternatives to injectable GLP-1 receptor agonists (GLP-1RAs).
The gastrointestinal tract initiates the release of GLP-1 from intestinal L cells in response to consumed nutrients like glucose and fatty acids [4]. GLP-1 plays a crucial role in maintaining stable blood sugar levels by promoting glucose-dependent insulin production from pancreatic beta cells and reducing glucagon output from pancreatic alpha cells [5]. This sophisticated feedback loop ensures effective regulation of blood sugar levels following a meal [6], [7].
GLP-1, beyond its role in glucose homeostasis, also controls hunger and promotes fullness by slowing stomach emptying and acting on hypothalamic neurons [8], [9], [10]. The therapeutic potential of GLP-1 has led to the development of GLP-1RAs, which mimic its effects [11]. These drugs have proven effective in managing chronic illnesses such as type 2 diabetes (T2D) and obesity, enhancing glycemic control, promoting weight loss, and reducing cardiovascular issues [12]. Clinical trials have consistently shown their benefits, including significant reductions in body fat and improvements in metabolic rate for obese individuals [13].
The secret of GLP-1RAs' medicinal efficacy is their complex interplay with several cellular signaling pathways. When it binds to the GLP-1R, a series of cellular reactions begin. Beta cell insulin production is enhanced by activation of pathways such as ERK1/2 and AMPK [14], [15]. Furthermore, Proglucagon (GCG) secretion is regulated by GLP-1 signaling through pathways that are dependent on cAMP [16]. It is worth noting that GLP-1 influences a complex network of signaling cascades, and recent investigations have shown that the MAPK and PKC pathways are also involved [17].
GLP-1's influence extends beyond metabolism to potential roles in brain diseases and certain cancers due to its ability to inhibit cell proliferation and promote apoptosis [18], [19]. MicroRNAs (miRNAs), which regulate gene expression, are emerging as promising modulators of GLP-1 signaling, offering potential for targeted treatments for conditions like T2D and obesity [20], [21], [22]. Injectable GLP-1RAs face limitations such as injection frequency and side effects, prompting the investigation of natural compounds that can modify GLP-1 function as alternative treatments.
This review aims to explore the physiological roles of GLP-1 and its inspiration for GLP-1RAs, delving into their therapeutic potential and the intricate signaling pathways they activate within cells, such as ERK1/2, AMPK, cAMP, MAPK, and PKC. It investigates the application of GLP-1RAs across various diseases, including cancer, and examines the role of miRNAs in regulating GLP-1 receptor expression. Additionally, the review considers the potential of natural compounds to regulate GLP-1 expression and secretion, offering alternatives to conventional medications with fewer side effects. By achieving these objectives, the review aims to highlight the remarkable versatility of GLP-1RAs, providing a deeper understanding of their mechanisms and paving the way for broader therapeutic applications.
2. Physiological roles of GLP-1
The GLP-1 is a 30-amino acid hormone that works mainly as an incretin in the β pancreatic cells, enhancing the release of insulin that is reliant on glucose. It is produced by the L-cells of the small intestine and proximal colon. Incretin mimetic medications known as GLP-1RAs improve glucose management by decreasing stomach emptying, decreasing GCG release, and enhancing insulin secretion and sensitivity [23].
By binding to the GLP-1R, GLP-1 is able to exercise its effects on a wide variety of cells and tissues. There is approximately 90 % sequence identity between the human GLP-1R and the rat GLP-1R, and the human GLP-1R [24]. Pancreas, brain, pituitary, stomach, heart, kidney, and hepatoportal area are among the many tissues where GLP-1R is expressed, which is in line with its wide range of activities. Some argue that GLP-1Rs are present in adipose tissue, skeletal muscle, the liver, and other tissues that insulin is thought to target [1]. Natural agonists of the GLP-1R include GLP-1(7−37) amide, and exendin-4 (9−39) (Ex-4), a GLP-1 mimic found in Gila monster saliva (Heloderma suspectum). At normal levels, the GLP-1R is not stimulated by structurally identical peptides such as GCG, GCG like peptide 2 (GLP-2), or GLP-1 breakdown products [25]. The intriguing oxyntomodulin (OXM), a peptide generated from proglucagon that is expressed in both the brain and the gut, may bind to the GLP-1R with a low affinity, allowing it to regulate hunger [26]. Many in vivo and in vitro studies make use of Ex-4, a well-known orthosteric antagonist for the GLP-1R [27]. It should come as no surprise that GLP-1 has pleiotropic effects beyond reducing glucose levels, considering the widespread distribution of GLP-1R. Among these effects are those on the heart and blood vessels, which include regulating hunger, inhibiting gastric acid secretion and gastric emptying, controlling glucose generation in the liver, and so on [28].
The broad tissue distribution of GLP-1 effects is in agreement with the ubiquitous expression of the GLP-1R. The endocrine pancreatic functions of GLP-1 have been the most thoroughly studied in terms of their physiological significance [29]. Beyond its role in pancreatic function, GLP-1 has several essential extraintestinal effects, such as regulating energy metabolism, improving nutritional absorption by the intestines, and stimulating the storage and utilization of nutrients in the liver, muscles, and fat [30]. Diuresis, lipid processing, brain function and repair, and cardiovascular repair and blood pressure regulation also seem to be important functions of this protein [31]. The release of insulin and somatostatin (SMS) are regulated by GLP-1 through the GLP-1R receptors found on islet β cells and δ cells, respectively. The release of GCG from islet α cells is inhibited by SMS through the SMS-2 receptor [32].
Beyond its role in blood sugar regulation, GLP-1 orchestrates the actions of other bodily organs [3]. Insulin secretion and GCG suppression are both controlled by it in the pancreas.
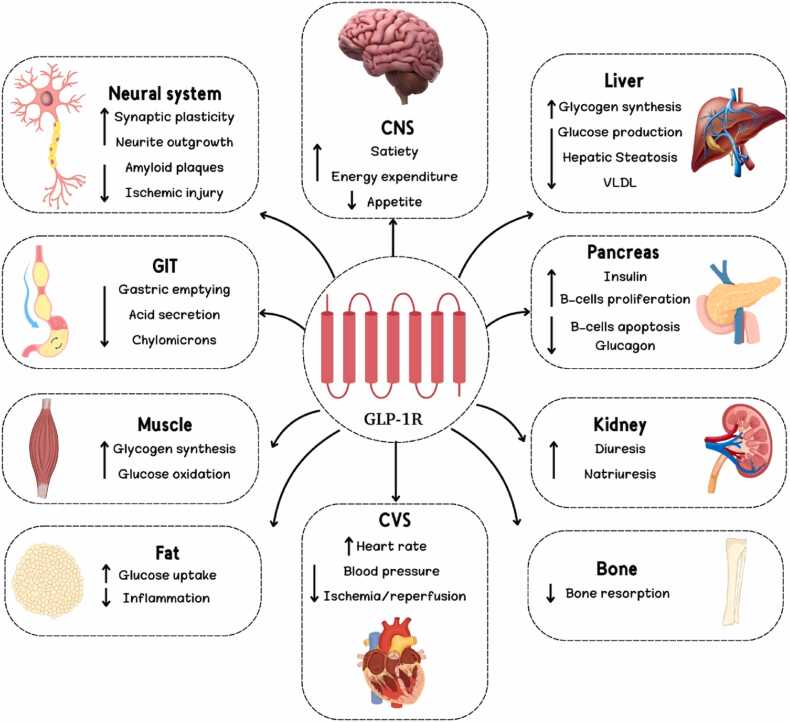
In order to decrease hunger and increase fullness, it sends signals to the hypothalamus, which regulates energy in the brain [33]. In the intestines, GLP-1 slows food flow and promotes nutrient absorption, much like a traffic cop. Muscles are also susceptible to its effects, which may aid in growth and insulin sensitivity [34]. GLP-1 promotes fat breakdown for energy and decreases storage, making it a fat sculptor [35]. Recent studies have shown that GLP-1 may have protective effects on the kidneys and heart, and its neural system localization raises the possibility that it plays a role in memory and learning [36]. Potential improvements in fat content and glucose production might also occur in the liver as a result of GLP-1's impact [37]. For T2D and maybe other diseases as well, GLP-1's pleiotropic characteristics make it an attractive treatment option [38]. A summary of GLP-1's pleiotropic effects is shown in Fig. 1[28].
Fig. 1.
Pleiotropic effects of GLP-1 [1]. [CNS: central nervous system; CVS: Cardiovascular system; GIT: Gastrointestinal tract; GLP-1R: Glucagon like peptide-1 receptor; VLDL: very low-density lipoprotein].
Based on the time effect and injection volume, GLP-1RAs can be categorized as either short-acting or long-acting. Beinaglutide and Exenatide are two examples of short-acting preparations that often require injection two or three times daily. Liraglutide and Lixisenatide are injectable, long-acting medications. Some long-acting formulations that are typically injected once weekly include Semaglutide, Dulaglutide, EX-LAR (long-acting release formulation of Exenatide), and PEX-168 (polyethylene glycol Loxenatide) [2].
All these GLP-1RAs agonists have different therapeutic effects including, reducing weight and inflammatory response, lowering blood glucose level-in type 2 diabetes mellitus, improving blood pressure, as well as, they have a cardioprotective and renal-protective effect [15]. In addition, GLP-1RAs have adverse effects including, hypoglycemia - especially when combined-, well tolerated most, GIT effects such as nausea, and vomiting, and other rare adverse effects [15].
3. Molecular mechanisms of GLP-1RAs
The intricate interaction between GLP-1RAs and other cellular signaling pathways is the key to their therapeutic effectiveness. The binding to the GLP-1 receptor initiates a cascade of biological responses. Activation of pathways including ERK1/2 and AMPK enhances beta cell insulin production [14], [15]. In addition, GLP-1 signaling regulates GCG secretion through cAMP-dependent pathways [16]. A series of interrelated signals causes GLP-1RAs to have their complicated metabolic effects. Notably, GLP-1 affects a web of signaling cascades; new research has revealed that MAPK and PKC pathways are also involved [17]. In the next section, we will discuss this complex interplay (Fig. 2).
Fig. 2.
The complex interplay between GLP-1RAs and several cellular signaling pathways. [AMPK: activated protein kinase; cAMP: cyclic adenine monophosphate; ERK 1/2: extracellular signal-regulated kinase 1/2; GLP-1R: Glucagon like peptide-1 receptor; MAPK: Mitogen-activated protein kinase; PKC: protein kinase C].
3.1. GLP-1 and ERK1/2 pathway
Multiple cellular functions benefit from ERK1/2 activation. MAPK signaling pathway, which includes ERK1/2, controls cell proliferation, differentiation, and transformation. Eukaryotic cells react to environmental changes through complex signal transduction networks. The three-tiered MAPK cascade is a common theme that converts extracellular cell-surface receptor activation into a specific biological response. Most research has focused on the ERK1/2 pathway [39].
Endometria from obese individuals had higher levels of ERK1/2 compared to normal-weight people, suggesting an association between obesity and enhanced endometrial cell proliferation and ERK1/2 activation. Likewise, endometria from overweight individuals exhibited elevated ERK1/2 phosphorylation when contrasted with normal-weight individuals [40].
Most research on diabetic cardiomyopathy shows that ERK1/2 activation impairs the heart's ability to endure oxidative stress, inflammation, remodeling, and apoptosis. ERK1/2 protects diabetics from myocardial infarction in addition to its pro-survival actions. Different stimuli cause ERK1/2 activity to different degrees, intracellular compartmentalization, and durations, suggesting that these effects are unique [41]. Insulin resistance (IR) decreases ERK1/2 activation in humans, according to recent studies. In an additional scenario, reduced ERK1/2 and MAPK activity hindered muscle glucose transport and caused insulin resistance (IR) [42]. After reviewing the research that established a connection between the GLP-1 and ERK pathways, we will go over a brief overview of the topic in Table 1.
Table 1.
Effect of GLP-1 on ERK signaling pathway.
| Pathway | Study Model | Medicine used | Tests applied | Results | Ref. |
|---|---|---|---|---|---|
| AKT/ERK | Rats | GLP−1 treatment. |
NOX3, SOD2, BCL−2, caspase3, LC3B, pAKT, pERK1/2, HDAC6 | The DR group had a substantial rise in pAKT and ERK1/2. | [43] |
| ERK1/2 | C57BL/6 mice | SA. | Insulin, OGTT, and plasma GLP−1 levels | Colon of SA-treated mice expressing pEKR1/2 and PC1/3. | [44] |
| MEK/ERK | Male KK/H1J mice. | Ezetimibe. | A test for insulin tolerance. The levels of GLP−1 in the blood and the intestines, mRNA expression in the intestines. | Ezetimibe induced MEK/ERK activation and a marked increase in active GLP−1 secretion. | [45] |
| PI3K/ AKT and ERK1/2 | Primary rat VSMCs. | Liraglutide | The CCK−8 was used to assess cell viability and proliferation. | Proliferation, AKT and ERK1/2 phosphorylation, and migration have all been enhanced. | [46] |
| AKT/ ERK1/2 |
Murine hippocampal HT22 cells. | GLP−1 treatment. | Hoechst 33342 staining of nuclear DNA. | Elevated levels of phosphorylated AKT and ERK1/2. | [47] |
This table represents the effect of GLP-1 on the ERK signaling pathway.
By reducing autophagy via the GLP-1R-ERK1/2-histone deacetylase 6 (HDAC6) signaling pathway, GLP-1 treatment enhances diabetic retinopathy (DR). There is a study investigates whether GLP-1 prevents cell death and autophagy in T2D rats' retinas. Previous studies have linked the protein kinase B (AKT)/ERK pathway to autophagy and apoptosis; this study examined this relationship. In retinal tissues from the ganglion cell layer, inner nuclear layer, and outer plexiform layer, phosphorylated AKT and ERK1/2 were considerably higher in the DR group than in the normal group. NADPH oxidase 3 (NOX3) and superoxide dismutase 2 (SOD2) levels were decreased by GLP-1 therapy. Treatment with GLP-1 in DR enhanced B-cell leukemia/lymphoma 2 protein (BCL-2) expression and decreased caspase3 and microtubule-associated proteins 1 A/1B light chain 3B (LC3B) levels. DR patients treated with GLP-1 had their GLP-1R expression restored, their levels of pAKT and pERK1/2 decreased, and their levels of HDAC6 were also reduced [43].
Another study used NCI-H716 cells and ERK1/2 inhibitors to investigate how Sennoside A (SA) induces GLP-1 synthesis. GLP-1 is secreted by intestinal cells to regulate blood glucose levels by promoting insulin production. One study demonstrated that SA elevated plasma GLP-1 in T2D mice. This study found SA improved mouse Oral glucose tolerance test (OGTT). SA significantly raised plasma GLP-1 and insulin. SA-treated animals also had increased colonic EKR1/2 and prohormone convertase 1/3 proteins (PC1/3) protein phosphorylation. The ERK1/2 inhibitor decreased SA-induced GLP-1 synthesis in NCI-H716 cells [44].
Ezetimibe's effects on glucose and GLP-1 secretion were examined. Participant insulin tolerance tests, serum and intestine GLP-1 levels, and GLP-1 gene messenger RNA (mRNA) expression were performed after 6 weeks of medication. In high fat diet (HFD) animals, ezetimibe significantly increased intestinally active and serum GLP-1. However, intestinal GLP-1 gene mRNA expression did not change. Authors examined ezetimibe's effects on L cell secretion and GLP-1 secretion in human NCI-H716 intestinal cells. Ezetimibe increased GLP-1 secretion and extracellular signal-regulated kinase kinase (MEK)/ ERK activation. Blocking the MEK/ ERK pathway with PD98059 eliminated ezetimibe's effect on GLP-1 secretion [45].
Whether Liraglutide mitigated the harmful effects of high-glucose (HG) therapy on lab-grown vascular smooth muscle cells (VSMCs) or not was examined. After exposure to HG, VSMCs increased migration, proliferation, and AKT and ERK1/2 phosphorylation. Liraglutide co-treatment considerably reduced these effects. Inhibiting phosphatidylinositol-3 kinase (PI3K) and ERK1/2 reduced HG's effects. GLP-1R inhibitors reversed Liraglutide's HG benefits [46].
Another study demonstrated that GLP-1-associated drugs alleviate T2D and improve Alzheimer's disease mouse pathology. After 1 hour of GLP-1 treatment, phosphorylated AKT and ERK1/2 levels were substantially greater than in vehicle-treated mice [47].
3.2. GLP-1 and cAMP pathway
GLP-1 acts via GLP-1Rs on various tissues, including the pancreas, to increase beta cell insulin synthesis and secretion and decrease alpha cell GCG release. Indirect effects of GLP-1 include decreasing hepatic gluconeogenesis and increasing neuroprotection in the liver and brain. GLP-1, gastrointestinal peptide, dipeptidyl peptidase 4 (DPP-4), G-protein coupled receptors (GPR)-119, and cAMP are acronyms [28].
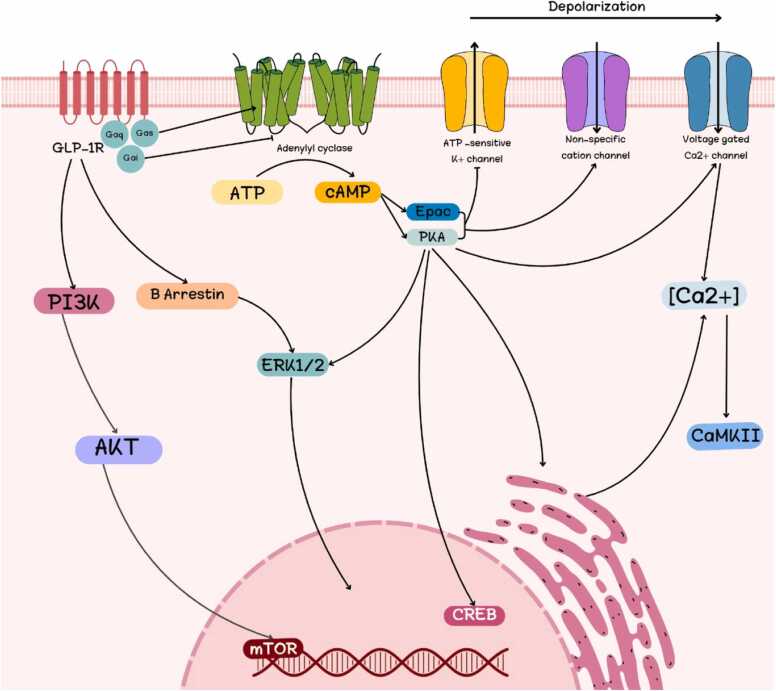
The process begins when GLP-1 binds to the GLP-1R. This activates adenylylate cyclase (AC), which increases cAMP. Then, protein kinase A (PKA) phosphorylates and activates cAMP response element-binding protein (CREB), a nuclear transcription factor that is constitutively expressed and involved in regulating the expression of genes related to neuronal survival and function. Initiating a cascade of cellular processes that culminate in insulin secretion and genetic modifications, GLP-1R-mediated stimulation of the cAMP/PKA pathway enables GLP-1 to exert its regulatory effects [28]. GLP-1 inhibits adenosine triphosphate (ATP)-regulated potassium (K+)channels, increases activity of L-type voltage gated calcium channels, and triggers opening of non-specific cation channels through PKA and exchange protein directly activated by cAMP (EPAC), two cAMP-dependent processes [48], [49]. Fig. 3 illustrates GLP-1R-mediated intracellular signaling. While GLP-1R has been linked to different G proteins, the most studied coupling is with Gαs. ERK1/2's activation-dependent effects show downstream singling’s intricacy. Upon activation, PKA temporarily moves to the nucleus, while β arrestin targets cytoplasmic partners [50].
Fig. 3.
Intracellular signaling through Gαs mediated by GLP-1R. [GLP-1R: Glucagon like peptide-1 receptor; ATP: adenosine triphosphate; cAMP: cyclic adenine monophosphate; Epac: exchange protein directly activated by cAMP; PKA: protein kinase A; ERK1/2: Extracellular signal-regulated kinase 1/2; AKT: protein kinase B; mTOR: Mammalian target of rapamycin; CREB: cAMP-response element binding protein].
Ligands binding to GLP-1R start a chain reaction that activates AC, a cell membrane enzyme, and generates cAMP. Activation of PKA or EPAC is often needed to start one of many signal transduction pathways downstream of cAMP synthesis. The GLP-1R pathway in pancreatic β-cells quickly increases glucose-dependent insulin release (in seconds to minutes). Directly activating PKA and EPAC raises cAMP quickly. These two cAMP signaling effectors synergistically increase insulin secretion in response to glucose stimulation by changing multiple secretory machinery targets [51], [52]. It turns out that insulin secretion is enhanced by a multi-process mechanism [1].
The process by which β-cells release insulin in response to glucose and GLP-1. GLP-1 boosts glucose efficacy by blocking K+ ATP channels, activating voltage-dependent calcium channels (VDCCs), and limiting membrane repolarization via K+ channels. GLP-1 also increases inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) and Ryanodine receptors (RYR) receptor sensitivity to Ca+ 2 effects, aiding Ca+ 2-induced Ca+ 2 release (CICR). CICR increases ATP synthesis, insulin secretion, and depolarization and secretion because insulin feeds back into the process [53].
In a study by Li et al., authors confirmed that Liraglutide enhances glucose transporter 4 (GLUT4) translocation via regulation of AMPK signaling pathways in mouse skeletal muscle cells. Liraglutide phosphorylated AMPK and increased intracellular cAMP. No changes were made to GLUT4 expression or AKT phosphorylation. Liraglutide raised intracellular cAMP and AMPK phosphorylation [54].
The AC activation and cAMP increase result from GLP-1 receptor binding. Next, cAMP-regulated PKA and EPAC2 operate. Depolarizing the membrane, activating the L-type VDCCs, and closing ATP-sensitive potassium (KATP) channels creates the action potential and calcium influx. PKA inhibiting delayed rectifying K+ channels lengthen action potentials. Another PKA effect is IP3 and RYR Ca+2 release. CICR is caused by EPAC2 activating the RYR and IP3R, which synthesize IP3 and diacylglycerol [55].
Increased cytoplasmic Ca+2 triggers mitochondrial ATP generation and insulin exocytosis from insulin granules. After meals, cAMP triggers GPR-119 receptors in intestinal L-cells to release GLP-1. GLP-1 and Gastric inhibitory polypeptide (GIP) help DPP-4 convert inactive metabolites quickly. DPP-4 inhibitors inhibit GLP-1 and GIP degradation [56].
Increased insulin production and secretion from beta cells and inhibition of GCG release from alpha cells in the pancreas are the primary effects of GLP-1 acting via certain GLP-1 receptors found on certain bodily tissues, including the pancreas. But GLP-1 also has an indirect effect on the liver (by lowering hepatic gluconeogenesis) and the brain (by increasing neuroprotection) [56].
After GLP-1 binds to its receptor, AC is activated, leading to an increase in cAMP levels and the subsequent activation of PKA. PKA activates L-type VDCC and closes KATP. Additionally, PKA causes the release of Ca+2 through RYR and IP3. As a result of an increase in cytoplasmic Ca+2 through all of these mechanisms, insulin is released exocytotically from insulin granules and mitochondrial ATP production is induced [56].
Obese mice treated with diethyl nitrosamine showed a considerable improvement in obesity-induced hyperlipidemia and hyperglycemia after receiving Exenatide. Additionally, the number of hepatocellular carcinomas in these mice was reduced, and the tumor cells themselves experienced death and repressed proliferation. Increased cAMP and PKA activation and GLP-1R upregulation were connected with Exenatide's tumor-suppressing actions [57].
Exenatide, completely prevented exenatide's stimulatory effects. The cAMP analogs cpt-cAMP and dibutyryl-cAMP replicated 3-isobutyl-1-methylxanthine (IBMX) and forskolin's anti-apoptotic activities, demonstrating that cAMP mediates these actions. It suggests that IBMX and forskolin's rapid protection did not require gene transcription or protein synthesis. The PKA inhibitors H-89 and KT5720 did not suppress these protective effects, indicating that they were not PKA-dependent. Attempts to determine this mechanism without PKA have indicated that GLP-1 and Exenatide produce low amounts of cAMP, while IBMX and forskolin produce higher cAMP, activating the cAMP pathway [58].
Subcutaneous injections of the GLP-1RA Ex-4 have been utilized in the management of T2D. Adiponectin was secreted into the 3T3-L1 adipocytes' culture media by Ex-4. The upregulation of adiponectin mRNA levels through the GLP-1R is responsible for this Ex-4 action. Preventing the stimulation of adiponectin expression by Ex-4 were both forskolin and IBMX, which may ultimately boost cAMP concentration [59].
Treatment with Liraglutide can enhance weight loss and improve lipid profiles in the blood. The breakdown of triglycerides (TGs) into free fatty acid and glycerin is known as lipolysis [60]. It has been proposed that hormone-sensitive lipase (HSL) in adipose tissue plays a crucial role in regulating lipolysis. The GLP-1R is activated when endogenous GLP-1 binds to it. PKA is activated when GLP-1R is activated because GLP-1R stimulates AC, which increases cAMP generation. HSL is phosphorylated by activated PKA [61]. Table 2 summarizes the effect of GLP-1 on cAMP signaling pathway.
Table 2.
Effect of GLP-1 on cAMP signaling pathway.
| Drug | Effect of cAMP on GLP-1R in diabetes | Animals used | Ref. |
|---|---|---|---|
| GLP−1-R | Raised levels of cAMP and insulin | Human pancreatic cell and rat INS−1 cell | [48] |
| GLP−1-R | Calcium ions are amplified | Mouse pancreatic cell | [49] |
| Liraglutide | The modulation of AMPK enhances the translocation of GLUT4. | Mouse skeletal muscle | [54] |
| Ex−4 | Direct induction of adiponectin expression is mediated by Ex−4. | Human | [59] |
| Liraglutide | Advantages of liraglutide for the treatment of obesity in older adults | Human | [60] |
| GLP−1 | The antiapoptotic action is mediated by GLP−1 through phosphorylation. | Human | [61] |
GLP-1: Glucagon-like peptide 1.
3.3. GLP-1 and AMPK pathway
The AMPK controls cellular energy balance. After metabolic stress, it promotes ATP-generating catabolic processes and inhibits ATP-consuming processes including cell growth and proliferation to restore energy balance [62]. The highly conserved master regulator of several metabolic pathways, AMPK, may be useful in treating obesity, non-alcoholic fatty liver disease and T2D [63]. AMPK overexpression reduces stress and cell death in β cells, vital for preventing type I diabetes (T1D). Clinical investigations have demonstrated that AMPK signaling improves diabetes consequences such brain problems. AMPK also improves diabetes- related neuropathy, nephropathy, liver disease, and reproductive changes. Providing protection [64].
The hypothalamus receives signals to enhance food intake through the AMPK pathway, which also inhibits insulin release from pancreatic β-cells. For a patient with T2D, practically every physiological outcome of peripheral AMPK activation would be helpful. Because of this, AMPK pharmacological activation has appeared to be an attractive target for medication development over the last 20 years [65].
The protein AMPK, which is heterotrimeric and includes catalytic α and regulatory β and γ subunits, is expressed everywhere and is controlled by various physiological signals and medications like metformin and thiazolidinediones. It is key in controlling processes like mitochondrial biogenesis, fatty acid synthesis, and glucose uptake [66], [67]. Following this, we will go over a few research that established a connection between the GLP-1 and ERK pathways, and then we will provide a brief overview in Table 3.
Table 3.
Effect of GLP-1 on AMPK, MAPK and PKC signalling pathway.
| Pathway | Drug | Effect | Ref. |
|---|---|---|---|
| AMPK signalling | Liraglutide | Dephosphorylation of mTOR and increased AMPK phosphorylation; decreased FBG and body weight | [68] |
| Exenatide | Initiated AMPK signalling, which in turn increased insulin signalling and fat oxidation enzyme activity. | [73] | |
| MAPK signalling | Liraglutide | Inhibits NF-κB pathway | [75] |
| Liraglutide | Increases the expressions of TNF-α, NF-κB and phosphorylated MAPK. | [77] | |
| Liraglutide | Inhibits activation of the NF-κβ and MAPK | [80] | |
| PKC signalling | Liraglutide | Up regulation of TRPC6 | [86] |
| Semaglutide | PKG/ PKC/ ERK1/2 pathway activation | [87] | |
| GLP−1RA | Inhibit PKCβ2 phosphorylation | [88] |
The link between AMPK and GLP-1-RA is recognized to improve cardiovascular health. Like metformin, liraglutide stimulates AMPK and lowers the mammalian target of rapamycin (mTOR) [68], [69]. Liraglutide significantly boosted AMPK activity in mice compared to the control group in diabetic ischemia-reperfusion research, suggesting it may stimulate the AMPK/mTOR signaling pathway. In diabetic mice undergoing ischemia-reperfusion, Liraglutide lowers fasting blood glucose (FBG) and body mass index (BMI) [68].
Long-acting GLP-1 analog liraglutide may improve endothelial function and enhance pro-angiogenesis and oxidative stress [70]. It may also activate AMPK. Exenatide increases AMPK phosphorylation and cAMP buildup in diabetic rats' hearts [69], [71]. Similar to the kidney, GLP-1 and its cleavage products reduce inflammatory cell invasion in mice to protect them against diabetic nephropathy [69], [72]. Recent study has shown that Exenatide and Liraglutide improve insulin sensitivity and lower fasting and postprandial blood glucose in T1D patients who do not make their own insulin. Exenatide and Liraglutide may modulate glucose homeostasis via AMPK without insulin, according to their research. They also show that Exenatide and Liraglutide directly increase AMPK-mediated skeletal muscle glucose absorption [67].
Short-term GLP-1RA Exenatide ameliorates intramyocellular lipid deposition without weight loss in obese mice (ob/ob) and diet-induced obese mice [73]. Treatment with exenatide increased insulin signaling pathway activity, AMPK signaling pathway activation, and lipid oxidation enzyme stimulation [73].
3.4. GLP-1 and MAPK pathway
The MAPK pathways are intricate signaling networks within cells that influence various functions, including regulating inflammation. One specific subgroup within the MAPK family, the p38 MAPK pathway, is activated by cellular stress factors and demonstrably linked to inflammatory processes. Studies have shown that activation of p38 MAPK coincides with increased inflammation in the kidneys, highlighting its potential role in inflammatory diseases. This in-depth analysis explores the potential link between chronic hyperglycemia, a hallmark of diabetes, and diabetic peripheral neuropathy (DPN), a devastating complication characterized by nerve damage. The p38 MAPK/ nuclear factor kappa B (NF-κB) pathway, a cellular signaling cascade known to be involved in inflammation, and its possible contribution to DPN [74], [75].
In streptozotocin-induced diabetic rats, Ma et al. verified that GLP-1RAs alleviate inflammation and peripheral nerve dysfunction through p38 MAPK/NF-κB signaling pathways [75].
Chronically high blood sugar levels, a defining characteristic of diabetes, may trigger inflammation via the p38 MAPK/NF-κB pathway. This specific pathway, when functioning normally, plays a crucial role in maintaining a balanced inflammatory response within the body. However, in the context of DPN, this pathway appears to malfunction, leading to the production of excessive inflammatory molecules. These inflammatory molecules can worsen diabetic complications, including nerve damage. Blocking this pathway could be a promising therapeutic strategy for DPN by curbing the inflammatory response and potentially preventing further nerve damage [76].
In a study by Ye et al., the protective effects of Liraglutide on glomerular podocytes in obese mice by inhibiting the inflammatory factor Tumor necrosis factor (TNF)-α-mediated NF-κB and MAPK pathway was examined [77]. The GLP-1RAs extend beyond glycemic control, the primary function of these drugs. GLP-1RAs play a role in reducing inflammation by inhibiting the p38 MAPK/NF-κB pathway. By blocking this pathway, GLP-1RAs might offer neuroprotection, safeguarding nerves from damage and potentially improving nerve function in patients with DPN. The p38 MAPK/NF-κB pathway as a potential target for DPN treatment [75], [77], [78].
When p38 MAPK is activated, it triggers a cascade of events that damage kidney cells and worsen inflammation. This pathway also activates the renin-angiotensin-aldosterone system, another contributor to kidney disease. Conversely, inhibiting p38 MAPK reduces inflammation and fibrosis, potentially improving kidney function [79]. The suppression of the NF-κB and MAPK pathways via GLP-1R is how Liraglutide reduces osteoclastogenesis [80]. There is some evidence that GLP-1 and GLP-1RAs can protect bone health. The possible cellular basis of the impact of Liraglutide on the differentiation of RAW264.7 preosteoclasts and murine bone marrow-derived macrophages (BMM) were examined. By boosting the expression of osteoclastogenic indicators following GLP-1R knockdown, the authors established that GLP-1R might be relevant for osteoclastogenesis and validated the presence of GLP-1R on BMMs and RAW264.7 cells. It is possible to prevent the production of osteoclasts and the resorption of bone by treating BMMs and RAW264.7 cells with Liraglutide. The NF-κB and MAPK signaling pathways were blocked by Liraglutide, which ultimately reduced the expression of nuclear factor of activated T cells (NFATc1). Moreover, the inhibitory impact of Liraglutide on NF-κB/MAPK-NFATc1 was nullified when GLP-1R was eliminated. Liraglutide inhibited receptor activator of NF-κB-induced osteoclastic differentiation [80]. Table 3 highlights research on GLP-1's impact on the MAPK signaling pathway.
3.5. GLP-1 and PKC pathway
According to Pujadas et al. [81], elevated glucose levels may cause an upregulation of PKCβ, which in turn may contribute to endothelial GLP-1 resistance by decreasing GLP-1R levels and interfering with the GLP-1 canonical route. Increased insulin secretion is induced by picomolar doses of GLP-1 through mechanisms that are dependent on PKC. GLP-1's capacity to induce phosphorylation in a PKC-dependent manner and the fact that the PKC activator can reproduce its effect on β cell electrical activity provide evidence that PKC is involved in GLP-1's stimulatory function [82]. One cause of the increased risk of myocardial infarction in diabetes is the development of resistance to GLP-1. The GLP-1 resistance in diabetic conditions is thought to be caused by the downregulation of the GLP-1R caused by PKCβ overexpression and the decreased GLP-1 post-receptor anti-apoptotic signaling pathways caused by PKCδ overexpression [83].
Research has shown that GLP-1 partially mediates the inhibitory effects on islet β-cell apoptosis via the PKC pathway [84]. At glucose concentrations below the stimulatory threshold, the GLP-1 may be essential for basal insulin production via a ternary signaling cascade including cAMP, PKA, Ca+2, and PKC [85]. Additionally, Liraglutide regulates the canonical transient receptor potential 6 (TRPC6), which is responsible for its reno-protective effects [86]. By stimulating the PKC/ ERK1/2 pathway, Semaglutide prevents cardiomyocyte apoptosis [87]. By blocking the activation of PKCβ2, GLP-1 significantly restored the damage caused by advanced glycation end products during the osteogenesis of human periodontal ligament stem cells (hPDLSCs) in vitro [88]. In Table 3, we can see how GLP-1 influences the PKC signaling pathway.
4. The role of GLP-1 in related diseases
4.1. Relationship between GLP-1 and diabetes
There is a local intestine intraepithelial lymphocyte- GLP-1R signaling network that regulates the mucosal immune response, and diabetes is marked by increased inflammation, which is indicative of problems in innate immune control [89]. GLP-1 agonists directly enhance insulin secretion by binding to GLP-1 receptors on pancreatic β cells. In addition to promoting hypoglycemic effects, they can increase the number of β cells in the pancreas, block cell death, and boost insulin production [90], [91]. In addition to increasing energy expenditure and motor activity, GLP-1RA has been found to activate brown fat metabolism in rats [32]. Another area of intense study right now is the impact of non-insulin glucose lowering medication side effects on weight reduction in T2D patients. Among several anti-diabetic medications, a systemic review found that GLP-1RAs and Tirzepatide were the most effective in causing weight loss in T2D individuals [92].
It was confirmed that GLP-1 increases insulin secretion from pancreatic β-cells in a way that is dependent on glucose levels, while simultaneously decreasing GCG production [93], [94], [95] Additionally, they enhance insulin sensitivity [96] and delay stomach emptying, which is useful in obesity [97].
For advantages beyond glycemic management, research has shown that it can aid weight loss by modulating signals that affect food intake. This makes it useful for treating obesity and T2D, both of which contribute to poor weight management [98].
There is a substantial decrease in severe adverse cardiovascular events like heart attack and stroke in individuals with T2D who use GLP-1 agonists, regardless of their impact on glucose levels. This suggests that GLP-1 may have cardiovascular protective properties. They provide a comprehensive strategy for cardiovascular health by enhancing vascular function, lipid profiles, and blood pressure [99].
Various studies have shown that GLP-1RAs have a kidney protective effect in patients with T2D, when considering the safety and effectiveness of GLP-1RAs in comparison to other anti-diabetic medications [100], [101], [102], [103], [104], [105]. If you compare GLP-1RA to other classes and medications, you'll see that it has a glycemic effect. Compared to other classes, GLP-1RAs are more effective in regulating blood sugar levels (Table 4).
Table 4.
Glycaemic effect of GLP-1 RAs compared to other antidiabetic drugs.
| Drug | HbA1c reduction | Ref. |
|---|---|---|
| Liraglutide | Decreased from 9.2 to 7.6 | [106] |
| Semaglutide vs placebo |
Decreased with Semaglutide | [107] |
| Oral Semaglutide vs Empagliflozin |
Reduced greater with oral Semaglutide at W 26 | [108] |
| Semaglutide vs Dulaglutide |
Reduced greater with Semaglutide than Dulaglutide | [109] |
| Vildagliptin vs Glimepiride |
Reduced greater with vildagliptin than Glimepiride | [110] |
4.2. Relationship between GLP-1 and obesity
Genetics, the environment, and other variables all have a role in the development of obesity, a complicated condition defined by the abnormal buildup of excess fat. Obesity is a major public health concern because of the rising rates of sickness, disability, and mortality it has caused in recent years, as well as the rising costs associated with treating these conditions [111]. Previous research has shown that reducing body fat can greatly lessen the likelihood of developing obesity-related problems and chronic diseases. To aid weight loss, the most common methods include dietary restrictions, moderate exercise, behavior modification programs, bariatric surgery, and medication treatment. Among these, anti-obesity medications are well-tolerated by patients and have a clear impact on lowering obesity rates in the short term. However, many of the medicines now used to combat obesity have limited clinical use due to concerns about their safety or effectiveness. There are many different organs that contain GLP-1RAs, which target the action of the incretin hormone and have their receptors in various parts of the body [112], [113]. Clinical trials and animal studies have shown that GLP-1RAs work better than other treatments for obesity. As a result, GLP-1RAs show great promise as obesity treatments [114]. Fig. 4 shows the potential processes by which GLP-1RAs induce weight loss [2].
Fig. 4.
GLP-1R: The Key to Unlocking Weight Loss? Exploring Potential Mechanisms [2].
How obesity causes a drop in GLP-1 secretion is still not well understood. One possible explanation for the drop in GLP-1 in obese people is an increase in plasma non-esterified fatty acids (NEFA), according to Ranganath [115] et al. The insulin-promoting impact of GLP-1, its putative role as a satiety factor, and greater fasting and postprandial NEFA levels may impede nutrition-mediated secretion, according to two clinical trials in patients with simple obesity [116]. Only a tiny subset of neurons in the central nervous system nucleus tractus solitarius express proglucagon and peptides produced from it, such as OXM, GLP-2, and proglucagon, GLP-1 [117].
To take part in energy balance, these make up the neuronal route that links the hypothalamus nucleus to the "visceral sensory" brainstem. Giving mice GLP-1, GLP-2, and OXM intraventricularly reduced their food consumption considerably [26].
A possible pharmaceutical technique to decrease food intake and maybe boost energy consumption could be the combination of central GLP-1 activation with GLP-2 and/or OXM activation [118]. Hypothalamic feeding centers express GLP-1 and/or GLP-1R, which has a direct impact on hunger and weight reduction [119], [120].
Furthermore, GLP-1 has the ability to decrease gastric acid secretion and postprandial gastric emptying, limit gastric and duodenal peristalsis through vagus nerve inhibition, and raise pylorus pressure, all of which contribute to a decrease in appetite, weight loss, and the so-called "ileal braking" effect [121]. Injecting Liraglutide into mice was done by Beiroa [122] et al. Research has shown that Liraglutide can promote browning of adipose cells and thermogenesis of brown adipose tissue even when no nutrients are consumed. It can decrease lipid content via the central GLP-1RAs' signaling pathway, which includes central AMPK or Sirtuin-1 (SIRT1) [122], [123], [124], [125], and increase thermogenesis and utilization of TGs-derived fatty acids and glucose in brown adipocytes.
4.3. Relationship between GLP-1 and cardiovascular diseases
Recent studies have shown that GLP-1R has a role in signal transduction and intracellular metabolism, and that it is highly expressed in human cardiovascular cells. These metabolites have direct or indirect protective effects on the cardiovascular system due to their biological activity, which can lower intravascular oxidative stress, inhibit hepatocyte gluconeogenesis and oxidative stress, boost cardiomyocyte activity, encourage vasodilation, and improve cardiac function [126], [127], [128]. For instance, as previously discussed, several large-scale clinical trials have shown that GLP-1RA may lower the incidence of cardiovascular events [129], [130].
4.4. Relationship between GLP-1 and nervous system disorders
There has been a recent emphasis on the GLP-1/GLP-1R axis' protective role against ischemic brain injury. Reducing ischemia-reperfusion injury, increasing brain healing, limiting inflammatory response, and oxidative stress are all ways in which GLP-1R activation can lessen the extent of cerebral infarction [131], [132], [133], [134]. GLP-1RA can alleviate neuroinflammation and behavioral abnormalities brought on by either neuropathic pain or hepatectomy [135], [136]. Additional benefits include a decreased risk of metabolic and cardiovascular diseases and a decrease in hunger due to GLP-1R activation in the brain [134].
4.5. Relationship between GLP-1 and cancers
There is still debate over the function of GLP-1RA in tumors. The next section of this review will discuss the interplay between GLP-1RAs and different types of cancers (Fig. 5 and Table 5)
Fig. 5.
The interplay between GLP-1RAs and different types of cancer.
Table 5.
Role of GLP-1RAs in different types of cancer.
| Cancer type | Result | Ref. |
|---|---|---|
| PC | The effects of Liraglutide on the migration, invasion, and proliferation of two human PC cell lines were investigated. Researchers created PC models in mice to find out whether Liraglutide GLP−1-based medications for T2D are beneficial or detrimental. The PI3K/AKT pathway is blocked by GLP−1RAs. | [147] |
| CRC | Among the numerous pleiotropic effects of GLP−1RAs are reduced plasma glucose, weight loss, and regulation of immunological function. An increased risk of CRC is associated with obesity. | [148] |
| CRC | Trial comparing insulin and metformin, two non-GLP−1RA anti-diabetics, with GLP−1RAs in patients with T2D. Potential effects of GLP−1RA on CRC risk | [149] |
| TC | A higher incidence of TC (any subtype) was observed in GLP−1 RA patients. An elevated risk of TC, particularly medullary TC, is linked to the use of GLP−1 RA. | [150] |
| PDACs | There was no independent predictive connection between GLP−1R expression and patients with PDAC. Possible molecular target for the detection and therapy of advanced prostate cancer is GLP−1Rs. | [151] |
| Prostate Cancer | Prostate cancer patient cell lines and tissue GLP−1R expression. By inhibiting the ERK/MAPK pathway, Ex−4 may impede the advancement of prostate cancer. | [143] |
| Prostate cancer | In vitro, primary human prostate cancer expresses GLP−1R, as does a cell line from lymph node metastases. One out of four patients had lesions that were avid for PSMA and Ex−4, indicating in vivo GLP−1R expression by human mCRPC. This study supports the use of GLP−1RAs to treat prostate cancer. | [152] |
| Prostate cancer | Prostate cancer cell proliferation was inhibited in vitro and in vivo by blocking cell cycle progression through GLP−1R forced expression. Both in vitro and in vivo studies have shown that overexpressed GLP−1R can impact prostate cancer. Some diabetic medications activate GLP−1R and slow the progression of prostate cancer. | [153] |
| BC | research subjects participating in the Liraglutide Clinical Development Program who were given GLP−1RA. Cancer risk rises with an elevated absolute risk of BC. | [154] |
| TC | The inhibition of two types of TC cell growth and metastasis was inhibited by Liraglutide-activated GLP−1R. Liraglutide offers a solid theoretical foundation for the treatment of diabetes. PI3K/AKT/mTOR pathway's influence on migration and proliferation of TC cells was counteracted | [155] |
Many worry that incretin-based therapy could lead to carcinogenesis, particularly pancreatic tumors, because of the stimulatory effects of GLP-1RA on β-cell proliferation and survival [137]. Elashoff found an elevated risk of pancreatitis and pancreatic cancer (PC) associated with treatment with DPP-4 inhibitor sitagliptin or GLP-1RA Exenatide compared to other medications by assessing the reported adverse events in the Food and Drug Administration (FDA) database. Patients treated with sitagliptin and those treated with other therapy had comparable rates of other cancers [138]. Another proven risk factor for PC, Exenatide can induce pancreatic duct hyperplasia, according to animal studies [139]. But there was no link between GLP-1RAs and an increased risk of cancer overall in a meta-analysis that included 37 relevant multiple dose-escalation. Treatment with albiglutide was associated with an even decreased risk of cancer overall in the subgroup analysis [140]. There was no increased risk of PC related with GLP-1RAs compared to other therapies, according to another meta-analysis that focused on PC [141]. Radiation therapy targeting insulinomas, which are known to express high levels of GLP-1R, has been facilitated by GLP-1R, which is an intriguing development. Using a mouse model of insulinoma, a single injection of labeled GLP-1RAs exhibited a dose-dependent reduction in tumor volume [142]. Theoretical investigations have shown that GLP-1RAs have the potential to slow the progression of prostate [143] and breast cancer (BC) [144], [145] tumors. All things considered, the precise function of GLP-1R in carcinogenesis is yet not known.
Obesity, IR, chronic inflammation, oxidative stress, and diminished adipokine imbalance are all pathophysiological hallmarks of both diabetes and cancer, although unlike diabetic angiopathies, diabetes does not hasten cancer's development or progression [146].
Treatments based on GLP-1, which are now used to treat diabetes by increasing insulin production and protecting pancreatic cells, may provide an unanticipated advantage for diabetic patients who have PC, despite the fact that obesity and IR increase the risk of both cancer and T2D. Despite early worries about safety, research has shown that GLP-1 medications may not only be safe for pancreatitis, but may also suppress the proliferation of cancer cells, giving them anti-tumor benefits. More study is required, but these findings suggest GLP-1 treatments may be useful for T2D patients with PC [147].
The FDA has approved GLP-1RAs for the treatment of T2D, according to Bendotti et al. Reduced plasma glucose, weight loss, and immune function modulation are only a few of the many pleiotropic effects of GLP-1RAs. Since being overweight or obese greatly increases the likelihood of developing colorectal cancer (CRC) [148]. In a statewide retrospective cohort research involving drug-naive patients with T2D, Currie et al. compared GLP-1RAs to seven non-GLP-1RA anti-diabetics, such as insulin and metformin, which are thought to affect the risk of CRC [149].
The use of GLP-1 RA was associated with a higher incidence of thyroid cancer (TC), specifically medullary TC, after one to three years of treatment, according to Bezin et al. The study found an elevated incidence of all TC among 2562 case patients with thyroid tumors and 45,184 control subjects [150].
Although immunohistochemical investigation could not identify GLP-1R expression as a separate prognostic factor in pancreatic ductal adenocarcinoma (PDAC) patients, Cases et al. (2015) noted that it does seem to have some bearing on the metastatic ability of PDAC. It is important to keep an eye on how GLP-1 mimic medicines fare in the long run and look into GLP-1R as a potential molecular target for advanced PDAC diagnostics and treatment [151].
The researchers Nomiyama et al. examined GLP-1R expression in prostate cancer cell lines and tissue samples from men and found that Exenatide might suppress the activation of ERK and MAPK, therefore reducing the progression of prostate cancer [143].
One of four subjects showed in vivo GLP-1R expression by human metastatic castration-resistant prostate cancer (mCRPC), avid for prostate specific membrane antigen (PSMA) and Ex-4, according to Stein et al. Now that we have shown that GLP-1R is expressed in osseous lesions from a single case of mCRPC and that a cell line derived from a human prostate cancer metastasis to a lymph node also expresses the GLP-1R in vitro, GLP-1R expression is present in all stages of human prostate cancer. The results add to the mounting body of evidence that suggests GLP-1RAs may be useful as a treatment for prostate cancer [152].
According to research by Shigeoka et al., GLP-1R inhibits cell cycle progression both in vitro and in vivo, which in turn reduces the growth of prostate cancer cells. As a result, activating GLP-1R could be a treatment option for prostate cancer. An effect on prostate cancer by activation of overexpressed GLP-1R in both in vitro and in vivo studies. Treatment options for prostate cancer may include diabetic medications that inhibit cancer progression and GLP-1R activation [153].
The development of medications for the treatment of obesity and diabetes is much concerned by Piccoli et al. over the potential risk of cancer. Subjects treated with a GLP-1RA had a greater absolute number of BC occurrences in the Randomized controlled trial of the Liraglutide Clinical Development Program [154].
The inhibitory effect was dose- and time-dependent, according to Zhang et al., who found that Liraglutide-activated GLP-1R could considerably decrease the development and metastasis of two types of TC cells. The PI3K/ AKT /mTOR pathway was suppressed in TC cell proliferation and migration by Liraglutide. Treatment of diabetes complicated with medullary TC might theoretically be based on this observation, which is comparatively safe [155]. To conclude, Table 5 summarizes the role of GLP-1RAs in different types of cancer
5. Role of miRNAs in regulating GLP-1R signaling
5.1. miRNAs and their role in diabetes and obesity
A family of tiny non-coding RNAs known as miRNAs is essential for controlling gene expression. They are primarily translated from deoxyribonucleic acid (DNA) and comprise 22 nucleotides, where typically, miRNAs induce mRNA degradation and translational repression by interacting with the 3′ untranslated region (3′ UTR) of target mRNAs [156]. The human genome contains mature miRNAs, and the public database miRBase v21 contains the transcripts' locations, sequences, and annotations. Over 2588 fully-grown miRNAs are encoded in the human genome. The whole set of miRNAs for a given genome is called the miRNome. Characteristics of the 5′ and 3′ sequences of miRNAs serve as borders, enclosing transcription start sites and recognition elements for transcription factors [157]. Approximately 50 % of miRNAs are expressed from introns of protein-coding transcripts. In certain cases, transcripts from the same locus or the sense and antisense strands of the same hairpin RNA undergo differential processing, resulting in miRNAs [158].
After the miRNA gene is translated by RNA polymerase II (Pol II), the first step in miRNA biosynthesis is the formation of primary miRNA (pri-miRNA). Then, precursor miRNA (pre-miRNA) is produced by splicing the stem loop miRNA molecule using the Drosha protein. A protein called exportin-5 facilitates the movement of pre-miRNA from the nucleus to the cytoplasm. After cutting the loop and degrading one strand, the Dicer protein splices pre-miRNA into the miRNA duplex molecule, resulting in the production of the mature, single-stranded miRNA molecule. After then, it becomes a part of the RNA-induced silencing complexes (RISC) complex, which is responsible for breaking down the mRNA target. Complexes like this are responsible for miRNA strand conversion from double to single strands, and the structure of miRNAs is what lets them attach to specific miRNA targets and control gene expression [159].
There have been reports of expression changes in several disorders, including T2D, and miRNAs can target more than one gene [160]. Many human bodily fluids, including as plasma, serum, urine, cerebrospinal fluid, saliva, and breast milk, include miRNAs in circulation. Biofluids contain miRNAs and other types of short RNA complexed with various components, one of which being extracellular vesicles [160]. Some have proposed miRNAs as biomarkers for predicting how well diabetic medications will work [161]. About half of all miRNAs are expressed from non-protein coding regions of the genome, and the other half are encoded in exons or overlapping introns. Similar regulatory processes, including epigenetic control and a regulatory feedback loop, impact miRNA expression as they do protein-coding genes. The importance of these tiny molecules in cell-cycle regulation, metabolism, apoptosis, stress responses, and cell differentiation and proliferation are being more and more recognized [162].
miRNAs biosynthesis and maturing pathway. Starting with the transcription of miRNA genes by Pol II, the biogenesis pathway ultimately produces mature miRNA molecules. primary miRNA (pri-miRNAs) with a stem-loop structure are responsible for miRNA transcription. Certain transcripts serve as targets for Pol II due to their 5′ cap structure and 3′ poly A tail [159]. Fig. 6 shows schematic representation of microRNA biogenesis.
Fig. 6.
A Schematic Representation of microRNA Biogenesis.
Increasing evidence suggests that miRNAs regulate many genes and participate in many molecular pathways. They may also disrupt immune system equilibrium by associating with immunity genes, which contributes to T1D pathogenesis. A substantial association exists between miRNA expression patterns and immune cell proliferation, activation, and differentiation. Damage and T1D begin when activated islet-specific T lymphocytes and antibodies target β-cells. This attack on the pancreatic islets involves cluster of differentiation CD4 + and CD8 + T cells, macrophages, natural killer calls, B lymphocytes, chemokines, and cytokines. Many immune responses may be affected by miRNA gene expression variations [159].
In animal models of T1D, miR-21 overexpression inhibits β-cell development [159], [163]. Exosomal miRNAs are involved in more than only physiological activities, including the beginning and progression of several diseases, including diabetes. It is becoming obvious that exosomal miRNAs are crucial to DM start and development. DM markers may be exosomal miRNAs released into the humoral circulation due to their sensitivity and specificity. Further research into exosomal miRNAs and DM processes will help us understand the physio-pathological process of DM [164].
Adipocyte differentiation and adipose tissue synthesis during adipogenesis are inhibited by Dicer deletion, a key miRNA processing enzyme. This suggests that miRNAs are involved. Low levels of adipocyte markers such peroxisome proliferator-activated receptor (PPARγ), TNF-receptor superfamily member 6, GLUT4, and fatty acid-binding protein 4 were observed [165], [166]. Adipocyte differentiation is controlled by miRNAs from preadipocyte development to terminal adipocyte differentiation, growth arrest, and clonal proliferation. miRNAs in the vascular endothelium may help alleviate obesity-related inflammation, capillary rarefaction, defective angiogenesis, and endothelial dysfunction [166].
5.2. Examples of miRNAs role in regulating GLP-1R signaling
The next section explores how miRNAs interact with GLP-1R signaling, influencing downstream cellular processes (Fig. 7 and Table 6).
Fig. 7.
Unveiling the hidden regulators: miRNAs and GLP-1R signaling.
Table 6.
miRNAs in regulating GLP-1 receptor signalling.
| miRNA | Targets | Function | Ref. |
|---|---|---|---|
| miR−7 | βARR1 cAMP |
GLP−1 mediates insulin secretion and miR−7 controls this process. While treatment with a particular inhibitor of miR−7 boosted cAMP synthesis, miR−7 reduced GLP−1-induced cAMP levels. | [167] |
| miR−204 | cAMP TXNIP |
Maintains agonist activity and GLP−1 receptor expression | [168] |
| miR−19b | ABCA1 | By reversing the miR−19b-induced downregulation of ABCA1, GLP−1 regulates cholesterol homeostasis. | [169] |
| miR−132 & miR−212 | cAMP PKA |
The expression of GLP−1 in pancreatic β-cells of both rodents and humans | [170] |
| mir−27a | AMPK PPAR-γ |
Through AMPK and miR−27a, GLP−1 ameliorated diabetic lung fibrosis. | [171] |
| mir−192 | β-cell |
By lowering GLP−1 expression, it controls the maturation of pancreatic beta cells and blocks insulin secretion. | [172] |
| miR−192–5p | intestinal microbiota | Control of the production of GLP−1 by L-cells in the intestines | [173] |
| miR−106b | β-catenin TCF4 |
Reducing miR−106b levels to enhance intestinal L-cell GLP−1 synthesis | [174] |
| miR−758 | ABCA1 | GLP−1 plays a role in ABCA1 via modulating cholesterol homeostasis by down-regulating miR−758. | [175] |
| miR−203a−3p & miR−429 |
Suppression of miR−203a−3p and miR−429 expression mediates the cardiovascular effects of GLP−1RA. | [176] | |
| miR−34a | SIRT1 | GLP−1 protects INS−1 cells from lipotoxicity in part through miR−34a, | [177] |
| miR−33 | GCG CREB1 FXR |
FXR inhibits GLP−1 secretion through miR−33 and its subsequent targets | [179] |
| miR−33a | Cnr1/CB1 GLP−1R |
Modifications to the miR−33a gene and the amounts of Cnr1/CB1 and GLP−1R mRNAs in the liver of T2D rats administered ghrelin. The levels of miR−33a are inversely correlated with Cnr1 and GLP−1R. | [180] |
| miR−23a | PGC−1α Bak Bax UCP2 |
Through downregulating miR−23a, GLP−1 boosted PGC−1α expression, which inhibited hepatocyte apoptosis. Additionally, GLP−1 improved UCP2 expression, which reduced apoptosis. | [181] |
| miR−23b | Elf4 | One factor that led to the development of obesity was the effect of WAT-derived exosomal miR−23b, which inhibited thermogenesis through regulating GLP−1R transcription by targeting Elf4. | [182] |
As an illustration, a study conducted by Matarese et al. [263] proved that miR-7 controls the release of insulin mediated by GLP-1 by aiming at β-Arrestin 1 (βARR1). Research has demonstrated that GLP-1 enhances the release of insulin in response to glucose via binding to the GLP-1R on pancreatic β cells. It is reported that βARR1 controls the desensitization of the GLP-1R. According to the authors, miRs have the ability to influence the GLP-1/βARR1 axis in β cells. In order to find miRs that potentially target βARR1, the authors utilized a bioinformatics technique. They succeeded in identifying miR-7 and validated its particular interaction with βARR1. They confirmed that GLP-1 could control miR-7 and βARR1 transcription, and that miR-7 played a substantial role in β cell cAMP synthesis and GLP-1-induced insulin release. While treatment with a particular inhibitor of miR-7 dramatically enhanced cAMP synthesis, miR-7 had a substantial negative effect on GLP-1-induced cAMP levels [167].
Research has shown that miR-204 regulates the production and action of GLP-1RAs. Previous research has shown that miR-204 inhibits the production of GLP-1RA in β-cells derived from rats, primary mice, and human islets by directly targeting its 3′ UTR. Additionally, protection against diabetes, increased glucose tolerance, cAMP generation, and insulin secretion were observed after in vivo deletion of miR-204, which elevated islet GLP-1RA expression and enhanced response to GLP-1RAs. Because thioredoxin-interacting protein (TXNIP) was shown to regulate miR-204 upstream. Another thing they did was see if TXNIP deletion in vivo may have the same effect as miR-204. Not only that, miR-204 improved glucose tolerance and insulin secretion triggered by GLP-1RA in islet GLP-1R expression [168].
According to research published in 2018 by Yao et al., GLP-1 regulates cholesterol levels via preventing the miR-19b-induced reduction of ATP-binding cassette transporter A1 (ABCA1). This work set out to discover how GLP-1 mitigates cholesterol-induced lipotoxicity in hepatocytes and what mechanisms are at play in this process. In this study, authors found that GLP-1 or HFD/cholesterol incubation altered miR-19b and ABCA1. GLP-1 had no effect on the PPAR-α protein, but it significantly increased the expression of ABCA1 protein. In groups treated with GLP-1, miR-19b levels were considerably reduced. The purpose of establishing miR-19b inhibition and overexpression was to investigate the impact of a miR-19b modification mediated by GLP-1. Cholesterol transport assays showed that GLP-1 administration improved ABCA1-dependent cholesterol efflux, leading to lower total cholesterol, either alone or in combination with miR-19b inhibitor. Additionally, the presence of lipid buildup was confirmed through histological analysis. Cholesterol reduced cell survival, increased hepatic cell apoptosis, and aided lipid buildup; however, GLP-1 had the opposite effect. According to the scientists, GLP-1 regulates the expression of miR-19b and ABCA1, which may impact cholesterol homeostasis [169].
Further research explored the impact of GLP-1 on miR-132 and miR-212 expression in human and mouse pancreatic β-cells. In a study investigated whether miRNAs affect GLP-1's glucose-stimulated insulin release, authors measured miRNA levels in rat insulinoma cell line (INS-1) cells and isolated islets from GLP-1-treated rats and humans using osmotic pumps in vitro and in vivo. By transfecting INS-1 cells with miRNA precursors or antisense inhibitors, researchers examined how miRNAs affect insulin secretion. GLP-1 upregulated miR-132 and miR-212 by more than 2-fold in INS-1 832/3 cells in rats, mice, and humans, as well as in vivo GLP-1-infused mouse islets. H-89, a cAMP-generating PKA inhibitor, decreased GLP-1's effects on miR-132 and miR-212. Despite weak cAMP and insulin responses to GLP-1, the 832/13 line of INS-1 cells did not increase miR-132 or miR-212 expression. Overexpressing miR-132 or miR-212 restored GLP-1 insulin responses in INS-1 832/13 cells and dramatically improved glucose-stimulated insulin production in 832/3 and 832/13 cells. GLP-1 increases miR-132 and miR-212 synthesis in pancreatic β-cells via a cAMP and PKA-dependent pathway. Overexpression of miR-132 or miR-212 increases glucose and GLP-1-stimulated insulin secretion [170].
GLP-1 reduced diabetic pulmonary fibrosis via AMPK and miR-27a, according to Liu et al., 2021. This study examined how GLP-1 influenced pulmonary fibrosis via AMPK/miR-27a. In HG medium, human embryonic lung fibroblast (MRC-5) cells were treated with miR-27a, GLP-1, and AMPK inhibitors. The cell counting Kit-8 (CCK-8) was used to count MRC-5 cells. The hyperglycemic group had significantly higher miR-27a expression and lower PPARγ expression compared to the control group. These proteins' expression can be reversed by miR-27a inhibitors. Time and concentration determined GLP-1's effect on miR-27a. After inhibiting AMPK, miR-27a expression increased. Using a projected Target Scan algorithm, miR-27a may target the PPARγ gene. Research shows that miR-27a targets PPARγ's 3′-UTR. Researchers found that miR-27a controls diabetic pulmonary fibrosis. GLP-1 may downregulate miR-27a via activating AMPK. Increasing the target gene PPARγ resulted in increased extracellular matrix proliferation in MRC-5 cells [171].
Another study indicated that miR-192 increases in T1D, affects pancreatic β-cell development, and reduces insulin production by lowering GLP-1 expression. This research aimed to investigate how miR-192 affects pancreatic β-cell development. In rats produced with T1D and streptozotocin, miR-192 levels were higher than in healthy individuals and normal rats. The miR-192 suppressed GLP-1 gene. Ectopic miR-192 production caused pancreatic beta-cell line NIT 1 cell death and lowered cell proliferation; however, miR-192 inhibitor had the opposite effect. In T1D, miR-192 is elevated and controls pancreatic β-cell growth by regulating cell proliferation and apoptosis, ultimately reducing insulin release. miR-192 also decreased GLP-1, promoting T1D. This study suggests treating and preventing T1D using miR-192 [172].
Liu et al. examined how chronic hepatitis B virus (HBV)- related gut microbiota imbalance affects miR-192–5p and GLP-1 expression in 2018. This study investigated how miR-192–5p and GLP-1 regulate gut microbiota dysregulation in HBV-infected diabetics. This study has three groups: HBV patients and alanine aminotransferase (ALT) levels. The gut flora of patients' excrement was examined. Each patient's feces, peripheral blood, and intestinal mucosal tissue were tested for miR-192–5p and GLP-1 expression. GLP-1 protein expression was also detected in intestinal mucosal tissue. HBV-positive patients with high ALT and low GLP-1 mRNA and protein expression had significantly increased miR-192–5p expression in intestinal mucosal tissue, peripheral blood, and feces. miR-192–5p mimics decreased GLP-1 expression whereas inhibitors increased it. Transfection of miR-192–5p precursors raised miR-192–5p and decreased GLP-1, whereas inhibitors significantly lowered miR-192–5p and enhanced GLP-1. These investigations found regulatory networks for intestinal microbiota imbalance, HBV infection, miR-192–5p, and GLP-1 expression [173].
Berberine increases intestinal L-cell GLP-1 production in vivo via stimulating the β-catenin/ T-cell factor 4 (TCF4) signaling pathway, as shown by Wang et al., 2021. Researchers found that mice fed a HFD with 100 mg/kg berberine daily had reduced miR-106b expression and elevated β-catenin and TCF4 expression in their colon tissues. Berberine also reduced tail vein blood glucose levels, boosted intestinal L cell GLP-1 synthesis in serum samples, and raised colon tissue GLP-1 expression in mice. Research revealed that 100 μM berberine decreased miR-106b expression in 293 T cells by raising methylation levels in the TCF4-linked miR-106b [174].
In their study on GLP-1 and cholesterol homeostasis, Yao et al. discovered that GLP-1 upregulates ABCA1 expression and downregulates miR-758. This study set out to investigate the mechanisms in HepG2 cells and find out how GLP-1 affects cholesterol-induced lipotoxicity in hepatocytes. Reversely, down-regulation of miR-758 intensified GLP-1's activity and demonstrated substantial promotion benefits, whereas overexpression of miR-758 abolished the GLP-1-mediated ABCA1 expression. Total cholesterol was lowered after treatment with an inhibitor of miR-758, which greatly improved ABCA1-dependent cholesterol export. These findings can help in the search for miR-758 biomarkers that target important lipid metabolism pathways [175].
Researchers Yao et al. found that GLP-1 regulates miR-758 expression and upregulates ABCA1 expression in their study on GLP-1 and cholesterol homeostasis [271]. Another study goal was to learn how GLP-1 influences cholesterol-induced lipotoxicity in hepatocytes by examining the underlying pathways in HepG2 cells. On the flip side, miR-758 down-regulation increased GLP-1 activity and showed significant promotion advantages, but miR-758 overexpression deleted GLP-1-mediated ABCA1 expression. Because it substantially enhanced ABCA1-dependent cholesterol export, a miR-758 inhibitor reduced total cholesterol. The identification of miR-758 biomarkers that specifically target critical pathways in lipid metabolism can be advanced by these results [176].
GLP-1 protects INS-1 cells against lipotoxicity in part because of miR-34a. Researchers set out to learn more about how miRNAs play a role in the GLP-1-mediated regulation of beta-cell activity. They measured cell viability and cell death. Research focused on the expression of genes related to beta-cell function, such as miR-34a and SIRT1. By conducting cell-transfection tests, authors dug further into the miR-34a underlying mechanisms. Palmitate dramatically reduced cell viability, enhanced cell apoptosis, activated miR-34a, and suppressed SIRT1 after incubating INS-1 cells for 24 hours. In addition to reducing palmitate-induced activation of miR-34a, co-incubation with GLP-1 protected the cells from palmitate-induced damage. In addition, cells infected with miR-34a mimics showed a large increase in palmitate-induced apoptosis, whereas those infected with miR-34a inhibitors showed a marked decrease. This provided more evidence that miR-34a plays a role in the GLP-1 mechanism that regulates the proliferation and survival of beta cells [177].
Farnesoid X receptor (FXR) interacts with CREB to inhibit intestinal L cell secretion of GLP-1 [178]. According to Li et al., FXR decreases GLP-1 synthesis through miR-33, GCG, and CREB1. Authors observed that FXR elevated miR-33. In addition, miR-33 targets stanniocalcin (intestinal neuroendocrine cell line (STC-1) cells and decreases GCG and CREB1 expression. Overexpression of FXR in STC-1 cells significantly reduced GCG, CREB1, and GLP-1 levels, while miR-33 inhibition had the opposite effect. The effects of overexpressing FXR were recovered by limiting miR-33 expression, demonstrating that FXR decreased GLP-1 secretion via boosting miR-33 expression and, by extension, GCG and CREB1 expression. These findings may improve T2D care [179].
Researchers Coskun et al. discovered that ghrelin-treated T2D rats' livers showed altered expression levels of cannabinoid receptor 1 (Cnr1/CB1) and GLP-1R mRNAs, as well as miR-33a and miR-122. Although ghrelin administration had no impact on blood lipid levels, TG, low-density lipoproteins (LDL), and very low-density lipoproteins (VLDL) levels were considerably higher in the T2D group as compared to control rats. In contrast to the control group, those with T2D had lower levels of Cnr1 and GLP-1R mRNA expression. In the T2D group that received ghrelin, these decreases were far more pronounced. The treatment group also showed less of an increase in miR-33a expression level compared to the T2D animals. Cnr1 and GLP-1R mRNA levels may have an inverse correlation with miR-33a expression levels, whereas miR-122 levels do not [180].
Wang et al. found that GLP-1 reduced hepatocyte apoptosis by upregulating miR-23a and uncoupling protein 2 (UCP2). GLP-1 increased the expression of the mitochondrial protective gene (PGC-1α). Diabetes and PGC-1α are closely linked. This HepG2 cell line study tested cell viability with glucotoxicity and GLP-1. The study examined gene expression alterations caused by hyperglycemia or GLP-1 by measuring RNA expression levels of miR-23a, PGC-1α, Bcl-2 antagonist killer 1 (Bak), Bcl-2- associated X protein (Bax), and UCP2. Additionally, PGC-1α protein levels were measured. To investigate miR-23a's role in PGC-1α regulation, cell transfection was employed to reduce its expression. The deleterious effects of hyperglycemia on viability were reversed by treating HepG2 hepatocytes with GLP-1 amide, which increased vitality and decreased Bax and Bak mRNA expression. After 24 hours of incubation with GLP-1 amide, miR-23a RNA expression decreased and PGC-1α mRNA and protein expression increased. High quantities of PGC-1α mRNA and protein were seen after inhibiting miR-23a expression through cell transfection. UCP2 mRNA expression increased after 24 hours of GLP-1 incubation [181].
Wang et al. showed that white adipocyte-derived exosomal miR-23b suppresses thermogenesis by targeting E26 transformation specific (ETS) transcription factor (Elf4). Brown adipose tissue (BAT) non-trembling thermogenesis and white adipose tissue (WAT) browning can prevent obesity. By feeding mice HFD, obesity was modeled. inguinal WAT (iWAT) and miR-23b antagomir exosomes were administered intraperitoneally. WAT-derived exosomal miR-23b increased BMI and IR. miR-23b suppressed Elf4 expression. Elf4 bound to GLP-1R's promoter to activate transcription. Adipocytes from BAT and iWAT indicated that GLP-1R knockdown restored WAT-derived exosomes' inhibitory influence on thermogenic gene expression and mitochondrial respiration, although miR-23b silencing had the reverse effect. WAT-derived exosomal miR-23b suppressed thermogenesis via targeting Elf4 to regulate GLP-1R transcription, contributing to obesity [182]. To conclude, Table 6 summarizes how miRNAs fine-tune GLP-1 receptor signaling.
The involvement of miRNAs, along with other exosomal or stem cell-specific miRNAs, expands the understanding of the diverse effects of various natural products on GLP-1 and contributes to the advancement of personalized medicine [183], [184].
6. Natural products and GLP-1 modulation
6.1. Why nature holds the key
The GLP-1 has revolutionized the way T2D is managed. Impressively, this incretin hormone promotes fullness, inhibits GCG secretion, and increases insulin secretion. Because of these effects, GLP-1RAs are a foundational treatment for diabetes and obesity. Nevertheless, the human body is not an island; a diverse array of natural substances can impact GLP-1 signaling, providing an intriguing opportunity for therapeutic investigation [12], [185].
Research into the possible modulation of GLP-1 activity is currently underway, focusing on plants that have been used for centuries in traditional medicine to control diabetes [186]. All the bioactive substances present in these plants are rekindling people's interest in them. To simulate the effects of synthetic medicines, some, such as curcumin and berberine, may directly activate GLP-1 receptors [187]. Cinnamon and fenugreek extracts, for example, may increase GLP-1 sensitivity and stability or boost its release from the intestines. A wider variety of therapeutic tools and avenues for potentially more tailored treatment approaches are made possible by this diversity of action mechanisms [188], [189].
Natural resources go beyond well-known therapeutic herbs. Green tea and soybeans are two examples of common foods whose bioactive components are also being studied [188]. This paves the way for promising new dietary therapies that may enhance current methods of treating diabetes. Imagine adding a cup of green tea to your daily routine together with medication, as it is high in GLP-1 stimulating characteristics [190], [191]. Another key benefit of natural goods over synthetic medications is their intrinsic safety profile. Well-tolerated medicines with minimal side effects are necessary for the long-term management of chronic illnesses such as T2D [190]. Patient compliance and quality of life could be greatly enhanced by using natural GLP-1 modulators, which may be easier on the body [192].
6.2. Why do we search for alternatives to GLP-1RA?
Synthetic GLP-1RAs have been incredibly successful, but there are still important reasons to look for alternatives [184]. There is a lot of worry about accessibility and cost. Patients who could benefit the most from these drugs may not be able to afford them [184], [193]. For populations with limited financial or other resources, natural remedies may provide a more accessible and less expensive alternative to conventional medicine [194].
One last thing to think about is the possibility of adverse effects. Although synthetic GLP-1RAs are usually well-tolerated, they do have the potential to induce gastrointestinal side effects including as diarrhea, vomiting, and nausea. Because of how annoying these side effects are, patients may not take their medication as prescribed [195]. Patients may have a more positive experience and get greater long-term results if they use natural products because they are less likely to have these negative effects [196].
Another important factor is the patient's preference. Many people believe that natural remedies are safer and better suited to a more holistic view of health, thus they may choose them over conventional medicine. People who are looking for a more natural approach to managing their diabetes typically find natural goods appealing [196]. Treatments for diabetes must also be easy to implement into daily life due to the long-term nature of the disease [197]. Incorporating natural products, especially those contained in regularly eaten foods, into dietary treatments could lead to a more holistic strategy for controlling blood sugar levels [196].
Finding GLP-1RA alternatives isn't about giving up on a successful medication; it's about increasing treatment options and personalizing it to each patient's preferences and needs. We can usher in a new age of GLP-1 regulation by tapping into nature's resources, giving patients more alternatives for managing T2D that are safe, effective, and well-tolerated.
6.3. Examples of natural products with modulatory effect on GLP-1
Natural products may modulate GLP-1 expression and secretion, according to some data. The present investigation will focus on components derived from herbs that have the potential to affect GLP-1 release, including berberine, curcumin, cinnamon, tea, and resveratrol [191]. In this part of the review, we will explore natural products that modulate GLP-1 secretion and expression (Fig. 8 and Table 7).
Fig. 8.
Exploring natural products that modulate GLP-1.
Table 7.
Natural products with modulatory effect on GLP-1.
| Compound | Nature | Source | Model used | Effect on GLP-1 | Ref. |
|---|---|---|---|---|---|
| Berberine | Isoquinoline Alkaloid | Roots, rhizomes, stems, and bark of certain plants such as Berberis | In vivo | promotes the release of GLP−1 by activating bitter taste receptors expressed in the intestines in a way that is reliant on PLC. | [191], [199] |
| Teadenol A | Polyphenol | Japanese post-fermented tea | In vivo | As a ligand of GPR−120, Teadenol A enhances GLP−1 secretion. | [200], [213] |
| Curcumin | Hydrophobic polyphenolic compound | Rhizomes of Curcuma longa |
In vivo & In vitro |
potential because it enhanced glycemic control by inducing GLP−1 secretion, making it a GLP−1 secretagogue. The enzymes PKC, ERK, and CaM kinase II were activated by curcumin |
[191], [201], [214] |
| Glycine and Cinnamon Extract | Glycine: Amino acid. Cinnamon: Spice. |
Glycine from most protein-rich foods. Cinnamomum zeylanicum. |
In vivo. | Plasma GLP−1 levels rise. | [202], [215] |
| Wheat Protein Hydrolysate | Grains | Wheat | In vivo | G protein-coupled receptor family C group 6 subtype A mediates LWP's stimulation of the Ca+2/CaM kinase II pathway, which substantially enhances GLP−1 production. | [209] |
| Gingerol | Beta-hydroxy ketone. | Zingiber officinale | In vivo | Treatment with gingerol increased plasma GLP−1 levels because it activated and upregulated cAMP, PKA, and CREB in the pancreatic islets. | [211] |
| Quercetin and its Glycosides | Flavonoid | Fruits, leaves, grains, and vegetables. | In Situ | Robust and sustained elevation of GLP−1 secretion with the Q3G + FOS test solution, in contrast to the transient elevation observed with the Q3G test solution and the trend for later-stage FOS test solution. | [191], [212] |
6.3.1. Berberine
Berberine activates the AMPK system, increases insulin production, decreases body weight and lipids, increases GLP-1, and lowers blood glucose [198]. Yu et al. investigated whether berberine-induced GLP-1 synthesis was linked to gastrointestinal bitter taste receptor activation. Authors showed that human enteroendocrine NCI-H716 cells exhibited bitter taste receptor subtype TAS2R38. TAS2Rs stimulation phenylthiocarbamide (PTC) was used as a positive control. NCI-H716 cells produced less GLP-1 after berberine treatment with anti-TAS2R38 antibody. Phospholipase C (PLC) and transient receptor potential ion channels (TRPM)5 inhibitors, which are implicated in bitter taste transduction, were employed to explore berberine-mediated GLP-1 production routes. U73122, a PLC inhibitor, reduced berberine-induced GLP-1 release in NCI-H716 cells, whereas quinine, a TRPM5 blocker, did not. Berberine-activated stomach bitter taste receptors to increase GLP-1 secretion in a PLC-dependent manner [199].
6.3.2. Teadenol A
Recently discovered from Japanese post-fermented tea, Teadenol A functions as a new ligand on a long-chain fatty acid receptor, GPR-120, according to research by Nagasawa et al., 2020. According to the results, Teadenol A directly binds to a ligand on a long-chain fatty acid receptor (GPR-120) and activates it. In addition, Teadenol A increased GLP-1 secretion by intestinal endocrine STC-1 cells. As a possible pharmacological target against T2D, GPR-120/GLP-1 signaling is gaining interest. Endocrine L cells in the small intestine are the primary sites of GPR-120 expression; these cells are responsible for sensing dietary fatty acids and activating GLP-1 release. Results showed that Teadenol A considerably increased GLP-1 production when administered to STC-1 cells. So, by binding to GPR-120, Teadenol A increases GLP-1 secretion [200].
6.3.3. Curcumin
Anti-glycemic curcumin has been shown in animals. Given the degradation products' potential for further effects, Alli-Oluwafuyi et al. explored their role in curcumin's anti-glycemic action in 2019. That study focused on curcumin's anti-hyperglycemic molecular mechanisms. The authors wanted to know if curcumin breakdown byproducts control GLP-1. The authors evaluated the effects of degradation-resistant and sensitive curcumin analogues on GLP-1 secretion. Curcumin, a pro-drug degradation product, helps the parent molecule fight hyperglycemia. The study examined GLP-1 release from mouse STC-1 cells, a primary source of this anti-diabetic hormone, after curcumin application. In both in vitro cell lines and rats, curcumin increased glycemic control via increasing GLP-1 production. Curcumin and its oxidative breakdown products activated PKC, ERK, and calcium/calmodulin-dependent protein kinase II (CaM kinase II), which secreted GLP-1. These data suggest curcumin's anti-glycemic effects are due to its oxidative metabolites [201].
6.3.4. Glycine and cinnamon extract
Glycine and cinnamon are known to have a positive effect on blood glucose levels [202]. One of the many physiological roles of the amino acid glycine is to boost immunity and anti-oxidative capacity; it is present in protein-rich meals [203]. Glycine, a nutritional supplement, has lately gained interest for its possible assistance in controlling blood glucose levels [204]. Cinnamon is a popular cooking spice that may have therapeutic uses as well, such as lowering blood sugar and blood pressure levels [205]. Isolated cinnamon components enhance insulin-dependent glucose metabolism, according to in vitro research [206]. Benefits associated with metabolic syndrome, including decreased FBG, have been shown in human studies using Cinnulin PF®, an extract of cinnamon [207].
In a study conducted by Bloomer et al. [202], ten participants, consisting of men and women, were seen to have elevated FBG levels. After a week-long interval, the participants were given a 25-gram glucose beverage with or without SugarClearTM, a proprietary combination of glycine and cinnamon extract, also known as Cinnulin PF®. The levels of glucose, insulin, and GLP-1 were measured in blood samples taken both before and after consumption. The overall area under the curve for GLP-1 rose. Enteroendocrine L cell line (GLUTag) studies have demonstrated that intracellular glycine increases GLP-1 production. Intestinal mucosal tissue contains these cells that are dedicated to producing GLP-1. Evidence suggests that Cinnulin PF®, a cinnamon extract that is water soluble and has a 20:1 ratio, can help with blood glucose control in several ways. After 22 people at risk of developing diabetes took Cinnulin PF® or a placebo for 12 weeks, Ziegenfuss and colleagues compared the two groups. FBG, systolic blood pressure, and lean muscle mass were all improved in the Cinnulin PF® group as compared to the placebo group [208]. When taken quickly after an oral glucose load, SugarClearTM—a patented combination of glycine and cinnamon extract—has a beneficial effect on insulin and blood glucose levels. Plasma GLP-1 levels are raised by the combination as well. For people whose blood glucose levels are consistently high, these changes could have beneficial metabolic effects [202].
6.3.5. Wheat
It has been claimed in several studies that wheat protein can help reduce hyperglycemia. One possible mechanism by which wheat protein hydrolysate lowers blood glucose concentration is via increasing the release of the hormone GLP-1. Using a GLUTag cell and studying the effects on glucose tolerance in rats through stimulation of GLP-1 secretion and subsequent induction of insulin secretion, Kato et al. determined whether wheat protein hydrolysate stimulates GLP-1 secretion and the molecular mechanism by which it does so. Compared to the high-molecular fraction of wheat protein hydrolysate, the low-molecular fraction of wheat protein hydrolysate (LWP) considerably enhanced GLP-1 production. The involvement of the Ca+2/ CaM kinase II pathway, which is mediated by G protein-coupled receptor family C group 6 subtype A, was determined to be responsible for this elevation. In addition, rats with hyperglycemia were able to have their symptoms alleviated by pre-administration of LWP, which stimulated GLP-1 production and induced insulin secretion [209].
Researchers Eelderink et al. looked at the variations in postprandial glucose kinetics and metabolic response after consuming fiber-rich breads with a high or low glycemic response by varying the wheat particle size. A randomized crossover trial was conducted with ten healthy male volunteers. The participants ate two types of 13C-enriched breads: one called kernel bread that had broken wheat kernels for 85 % of the flour, and another called control bread that had wheat flour and wheat bran as its ingredients. Compared to control bread, the GLP-1 response after kernel bread was noticeably lower. Bread made with 85 percent broken kernels instead of whole wheat flour had a significantly different GLP-1 response but no effect on glucose response or kinetics. Hence, altering the wheat's processing parameters before bread baking can affect health by influencing the metabolic response beyond glycemia [210].
6.3.6. Ginger
Zingiber, or ginger, is a popular culinary spice and well-known medicinal herb. New research suggests that ginger and its chemical constituent gingerol may induce GLP-1 production, which in turn has a hypoglycemic impact, explaining why ginger has long been prescribed to diabetic patients [191].
Ginger root's 6-gingerol reduces hyperglycemia in T2D mice. Samad et al. examined how 6-Gingerol reduces hyperglycemia in Leprdb/db diabetic mice and whether it effects insulin secretion via the endocrine route. 6-Gingerol was given orally to Leprdb/db T2D mice daily for 28 days. Fasting and fed endocrine hormone plasma levels were measured. GLP-1 was controlled using drugs. 4-weeks of 6-gingerol treatment increased glucose tolerance and glucose-stimulated insulin production. Animal plasma GLP-1 levels increased significantly after treatment. Drug modification of GLP-1 levels by 6-Gingerol regulated insulin production. 6-Gingerol mechanistically activated cAMP, PKA, and CREB in pancreatic islets, which are essential to the GLP-1-mediated insulin secretion pathway [211].
6.3.7. Quercetin and its glycosides
A polar auxin transport inhibitor, quercetin is a flavonoid present in many plant foods including fruits, leaves, cereals, and vegetables. There is some evidence that the glycoside derivatives of quercetin, like rutin, can enhance the glycemic profile and increase insulin sensitivity. According to some research, GLP-1 secretion regulates these hypoglycemic effects [191].
The 2014 study by Phuwamongkolwiwat et al. sought to examine the effects of fructo-oligosaccharide (FOS) and quercetin-3-O-β-glucoside (Q3G) on metabolic syndrome indices and plasma total cholesterol level, as well as their individual and combined effects, and the possible mechanisms of action. After ileal injection, rats were used to test the effects of Q3G and FOS on portal GLP-1 secretion independently. The authors observed that the GLP-1 secretion was significantly and persistently increased with the Q3G + FOS test solution, in contrast to the temporary increase observed with the Q3G test solution and the trend for later-phase increases observed with the FOS test solution. Research suggests that FOS-induced GLP-1 release may help diabetic rats with insulin secretion and hypoglycemia. Hence, Q3G + FOS may help reduce IR and plasma glucose concentration by increasing GLP-1 production [212]. To sum up, Table 7 summarizes natural products with modulatory effects on GLP-1.
7. Alternative therapies to GLP-1 receptor agonists
Based on the time effect and injection volume, GLP-1RAs can be categorized as either short-acting or long-acting. Beinaglutide and Exenatide are two examples of short-acting preparations that often require injections two or three times daily. Liraglutide and Lixisenatide are injectable, long-acting medications. Some long-acting formulations that are typically injected once weekly include Semaglutide, Dulaglutide, EX-LAR (long-acting release formulation of Exenatide), and PEX-168 (polyethylene glycol Loxenatide) [2].
7.1. Short−acting GLP−1 receptor agonists
7.1.1. Beinaglutide
Genetically engineered Beinaglutide has the same amino acid sequence as human GLP-1. Approved for T2D hypoglycemic intervention and indicated for meal injection, it helps patients whose blood glucose control is poor with metformin alone. Beinaglutide alone improves blood glucose management. The recommended dosage is 0.2 mg (100 µl) three times a day after the first two weeks of treatment. With an 11-minute half-life, this medication regulates blood glucose levels within 2 hours of eating. No drug buildup occurs since it is quickly removed [216]. Wang et al. enrolled 36 T2D patients with a body mass index (BMI) of 24 kg/m2 or higher in their clinical investigations. Several biomarkers showed a marked decline after three months of Beinaglutide treatment, including BMI, fasting blood glucose (FBG), glycated hemoglobin (HbA1c), visceral and subcutaneous fat, leptin, C-reactive protein, and tumor necrosis factor (TNF) [217]. Fang et al. tested Beinaglutide on C57BL/6 and ob/ob mice for pharmacological and pharmacokinetic effects. In glucose tolerance tests, Beinaglutide dose-dependently increased insulin secretion, lowered glucose levels, and inhibited meal intake and stomach emptying. Beinaglutide's ability to restrict meal intake for over four hours at higher doses caused ob/ob mice to lose weight after two weeks of therapy. In the nonalcoholic steatohepatitis (NASH) model, Beinaglutide reduces liver weight and steatosis and regulates insulin. Changes were seen in mitochondrial function, antioxidant, and sirtuin-1 (SIRT1). This suggests that Beinaglutide may cure obesity and NASH [218]. In a clinical trial of 78 non-diabetic adults, Beinaglutide may be better than metformin for treating obesity [219]. An in vivo study found that Beinaglutide reduces obesity produced by a high fat diet (HFD) by affecting lipid metabolism genes and adipose tissue lipid classes [220].
Beinaglutide reduces weight, controls inflammation, and is cleared by the kidneys with fewer side effects [2].
7.1.2. Exenatide
The first GLP-1RA approved for T2D is Exenatide. The Gila monster’s saliva contains a synthetic version of a hormone called Ex-4. Ex-4 has hypoglycemic effects such as lowering hunger, boosting insulin production, blocking α cell GCG release, and slowing gastric emptying. In function, it's like human GLP-1 [221], [222].
Exenatide exhibited delayed kinetics due to its resistance to dipeptidyl peptidase 4 (DPP-4) proteolysis, in contrast to human GLP-1, which was rapidly destroyed by DPP-4 [223]. Exenatide is detectable in plasma as early as 15 minutes following delivery and remains detectable up to 15 hours following a single subcutaneous injection at a dose greater than 0.2 µg/kg, due to its relatively brief half-life of 2.4 hours [221].
Obesity-related lipotoxicity from saturated free fatty acids damages kidneys. Exenatide repairs renal tubular epithelial cells damaged by a HFD by reversing SIRT1 downregulation, inhibiting reactive oxygen species, and blocking mitochondrial apoptosis [224], [225].
Clinical studies using Exenatide for T2D are more common than those for obesity. Research on healthy and diabetic individuals indicated that Exenatide alone improved control of HbA1c, fasting and postprandial blood glucose levels, weight loss, β cell activity, and blood pressure [226].
In a 30-week triple-blind, placebo-controlled clinical investigation, Exenatide caused much more weight loss than placebo. Participants in the research had T2D and a BMI of 33 ± 6 kg/m2. The most prevalent side effect of Exenatide is nausea, which may explain its weight loss effects [227]. Apovian conducted a 24-week RCT that found Exenatide plus lifestyle changes lowered participants' weights by 6.16 kg. However, nausea was more prevalent than in the control group [228].
Results show that vomiting is still the most common adverse reaction in 152 cases of simple obesity (5.1 kg weight loss), 60 cases of polycystic ovary syndrome (PCO) (3.2–6.0 kg weight loss), and 10 cases of metabolic syndrome (3.7 kg weight loss) studied with Exenatide for non-diabetic obesity [229], [230], [231].
Finally, Exenatide helps reduce weight in diabetics regardless of weight. It can also help overweight people with basic obesity and PCO/metabolic syndrome lose weight. Clinical studies suggest it may assist overweight non-diabetics lose weight. Injectable therapy can cause vomiting and is inconvenient [232].
7.2. Long−acting GLP−1 receptor agonists: once-daily
7.2.1. Lixisenatide
As a GLP-1RA, Lixisenatide is taken daily. The synthetic counterpart of Ex-4, Lixisenatide, improved its circulation half-life and GLP-1R binding affinity by four by replacing one proline with six lysines. The slow dissociation rate and significant receptor affinity prolong the pharmacological effect [233], [234]. Patients with T2D should reduce their fasting and postprandial blood glucose levels in order to effectively lower HbA1c levels [235], [236]. Lixisenatide is rapidly absorbed into the bloodstream after subcutaneous treatment; proteolytic enzymes break it down and the kidneys eliminate it. The liver does not biotransform it. Besides lowering blood glucose, Lixisenatide improves cardiovascular health, delays stomach emptying, protects pancreatic beta-cells, and increases insulin messenger RNA (mRNA) expression and hormone release [237], [238]. Furthermore, Lixisenatide has a low incidence of side effects, with the most prevalent ones being vomiting and nausea [239], [240].
A prospective clinical trial conducted in Spain discovered that Lixisenatide has the ability to improve blood lipids, particularly total cholesterol and triglycerides (TGs), in addition to managing glucose levels in the blood (HbA1C and FBG) [241]. It is possible that Lixisenatide lowers chylomicron TGs via increasing clearance, which delays gastric emptying [242].
Compared to the iGlar trial (insulin glargine U100), Japanese adults with T2D lost more weight in the iGlarLixi study (insulin glargine: Lixisenatide = 1:1). In this 26-week RCT, iGlarLixi participants had more gastrointestinal side effects, mostly nausea [243].
When basic insulin treatment failed, Lixisenatide plus additional diabetic drugs caused a 0.9-kg weight reduction and a 1.3-kg improvement in HbA1c and FBG [244]. Lixisenatide considerably decreased patients' body weight [245], [246], [247]. Nausea and vomiting are the most common side effects of Lixisenatide in the first stage of gastrointestinal adverse reactions. Lixisenatide has fewer gastrointestinal side effects and hypoglycemic episodes, and it requires less injections under the skin than Exenatide. While both Lixisenatide and Liraglutide are well-tolerated, the former is superior at lowering weight and improving blood glucose levels [248], [249], [250], [251].
Clinical trials show that Lixisenatide is well-tolerated and can help overweight people lose weight. Since there are no differences in tolerance, efficacy on glycemic indices, weight reduction, or administration benefits, Lixisenatide will likely be substituted for other GLP-1 agonists for financial reasons [252].
7.2.2. Liraglutide
By gene recombination, liraglutide was created by adding a 16-carbon palmitoyl fatty acid to the 26th position of GLP-1 and replacing the 34th lysine with arginine. These structural alterations increase albumin aggregation and non-covalent binding, blocking subcutaneously administered isotonic fluid DPP-4. Liraglutide administration is recommended once daily, with a Tmax of 9–13 hours and t1/2 of 13 hours. Weight loss occurs at 3 mg/day, which is its principal clinical application for T2D [253], [254], [255].
It is an obesity treatment that has received approval from the Food and Drug Administration (FDA). Its basic concepts include the following: it may reduce hunger by inhibiting the feeding center; it can slow digestion and absorption of food by delaying stomach emptying; and it can achieve the role of diet control [256], [257], [258].
Liraglutide is more effective in reducing FBG and HbA1c than short-acting GLP-1RAs. Compared to twice-daily Exenatide, it has fewer negative effects [259].
In comparison to orlistat (3 ×120 mg, 4.1 kg weight loss), Liraglutide (1.2 mg/d, 1.8 mg/d, 2.4 mg/d, or 3 mg/d) resulted in an average weight loss of 4.8–7.2 kg in a clinical study including 564 adults with a BMI of 30–40 kg/m2 who did not have T2D. The results of the two-year extended experiment were similar [260], [261]. Those who had nausea or vomiting while using Liraglutide 3.0 mg lost 9.2 kg per year, compared to 6.3 kg for those who did not. Nausea and vomiting increase with weight loss [262]. Patients with obesity and prediabetes can benefit from taking Liraglutide 3 mg for three years since it reduces their risk of diabetes [263].
Pi-Sunyer et al. gave 3731 non-diabetics with a BMI of 27 kg/m2 or 30 kg/m2 3 mg of Liraglutide subcutaneously once a day. After 56 weeks of treatment, mild to moderate nausea and diarrhea were the most common side effects, resulting in an average weight loss of 8.40 ± 7.3 kg [264]. In the 56-week research, 422 non-diabetic patients with BMIs of 30 or 27 kg/m2 and dyslipidemia or hypertension were recruited. These individuals lost at least 5 % of their baseline weight on a low-calorie diet for 4–12 weeks before the experiment. Researchers expected that Liraglutide 3.0 mg/d will improve weight loss maintenance since more Liraglutide patients maintained off at least 5 % of their lost weight than placebo [265].
Clinical trials for the treatment of diabetes and obesity have included GLP-1 analogs, which share 97 % sequence identity with human GLP-1. It should be mentioned, nevertheless, that when taken with insulin, it can cause hypoglycemia [266].
7.3. Long-acting GLP−1 receptor agonists: once-weekly
7.3.1. Semaglutide
Semaglutide, a long-acting GLP-1 analog, manages T2D and helps moderately obese people lose weight [267], [268]. Obesity treatment involves weekly subcutaneous injections. The drug may cause weight reduction in diabetics and non-diabetics [269], [270]. This review focuses on synthesized Semaglutide therapy impact in obesity (STEP) trials.
In the STEP2 research, nearly 1200 obese T2D were given 1 mg, 2.4 mg, or a placebo monthly. One treatment group lost −6.9 kg (-7 %) and the other −9.7 kg (-9.6 %). Semaglutide 2.4 mg once weekly can help overweight or obese T2D lose weight, as the 2.4 mg group lost the most [271]. In the STEP1 experiment [272], 1961 people without diabetes and a BMI of 30 kg/m2 (or ≥27 kg/m2 with weight-related illnesses) were enrolled. Semaglutide (2.4 mg once weekly) caused 15.3 kg of weight loss after 68 weeks. Semaglutide patients lost more than placebo patients [273], [274].
Semaglutide is available in oral and injectable versions in the United States, although only the injectable form is approved for obesity [275]. GLP-1RAs like Semaglutide cause many adverse effects. The most prevalent gastrointestinal side effects were nausea, diarrhea, and vomiting. Most people report mild to severe side effects that subside [272], [273].
7.3.2. Dulaglutide
Long-acting GLP-1RA dulaglutide reduces hunger, slows stomach emptying, and boosts insulin secretion. It is approved to treat T2D hyperglycemia in numerous countries [276], [277]. Dulaglutide, like other GLP-1RAs, leads to weight loss or reduced gain alone or with non-insulin secretagogues [278]. Weekly injections have a long half-life. Subcutaneous injections work best within 48 hours. The most prevalent side effects were diarrhea, vomiting, nausea, and headache [279]. Most first-time drug users experienced mild to moderate gastrointestinal adverse effects [280].
Weekly Dulaglutide versus daily Liraglutide in metformin-treated uncontrolled T2D patients is compared for safety and efficacy. Dulaglutide subcutaneously injected weekly loses weight like Liraglutide once a day: −2.90 kg at 26 weeks (-3.61 kg with Liraglutide) [280]. At 26 weeks post-treatment, 1.5 mg Dulaglutide caused weight loss of −2.29 ± 0.24 kg, 0.75 mg Dulaglutide −1.36 ± 0.24 kg, and metformin −2.22 ± 0.24 kg [281]. Dulaglutide can be increased from 1.5 mg to 3.0 mg or 4.5 mg in T2D not well managed by metformin to achieve HbA1c and weight reduction comparable to clinically relevant levels with the same safety [282]. In the AWARD-2 study, Dulaglutide caused numerous pancreatitis episodes [283]. Thus, pancreatitis symptoms must be continuously monitored when taking Dulaglutide. Pancreatitis requires immediate medication discontinuation [284].
8. Recent advancements in GLP-1-based therapies
8.1. Long-acting release formulation of exenatide (EX-LAR)
The FDA-approved polylactic-co-glycolic acid is a common microsphere synthesis polymer due to its biodegradability and safety. It is often used as a biological scaffold and drug carrier. Exenatide can be released slowly in microspheres. After weekly subcutaneous injections, diffusion and microsphere rupture released Exenatide microspheres into the bloodstream over 6–8 weeks, allowing the medicine to reach a stable plasma concentration. EX-LAR reduced nausea and vomiting compared to Exenatide [285].
A non-inferiority RCT compared 295 T2D patients using 2 mg of EX-LAR weekly with 10 µg of Exenatide twice daily for 30 weeks. Weight loss rates were similar for Exenatide and EX-LAR [286]. In a study of 134 T2D treated for 104 or 117 weeks, those who had never received Exenatide microspheres lost 2.7 kg [287]. Elkind-Hirsch et al. found that non-diabetic women with PCO and a BMI of 30 kg/m2 or less and 45 kg/m2 or less lost weight following 24 weeks of weekly EX-LAR or Dapagliflozin. Weight loss was greatest with double therapy [288].
The EX-LAR treatment weekly may be a promising diabetes prevention method, considering the favorable effects of GLP-1R activation on β cells and weight loss. Patients without T2D or impaired glucose tolerance can potentially benefit from weekly EX-LAR preparation for obesity. Few scientific trials have examined EX-LAR for weight loss, and most involve T2D patients [289].
8.2. Polyethylene glycol Loxenatide (PEX-168)
The early GLP-1RA takes repeated injections and has a high rate of digestive system side effects, limiting its use. After amino acid alteration and PEGylation from Exenatide, PEX-168 is the first GLP-1RA with long-term pharmacological efficacy [290]. A new long-acting GLP-1RA. Long intervals between dosages (one weekly), low gastrointestinal side effects, and moderate to mild side effects are benefits [291].
Guo [292] et al. found that PEX-168 significantly reduced basic obese mice's weight. The low-dose (0.03 mg/kg), medium-dose (0.1 mg/kg), and high-dose (0.33 mg/kg) groups lost 2 g, 4 g, and 1 g of weight after 8 weeks. After four weeks of PEX-168 therapy at various doses, a multicenter, multiple dose-escalation RCT demonstrated weight reduction of −0.8–1.8 kg. Therapy caused a 1.4–3.3 kg weight decrease after 8 weeks [291].
8.3. Others: GIPR/GLP-1R dual agonists and GIPR/GLP-1R/GCGR triagonists
Gastric inhibitory polypeptide (GIP) is produced by intestinal endocrine K cells. Like GLP-1, GIP triggers insulin release from pancreatic β cells, although glucose is required [293]. Low blood glucose levels trigger the release of GCG by pancreatic α cells. It has been known to increase energy expenditure for over 60 years [294]. Recent clinical trials for T2D and obesity have utilized multi-targeting GIP receptor (GIPR), GLP-1R, or GCG receptor (GCGR) agonists. These agonists aim to maximize metabolic benefits and minimize side effects. Thus, developing appropriate drugs and replacing double and triple GIP-GLP-1RAs like tirzepatide and peptide 20 are crucial [295].
Studies on overweight mice show that GCGR activation distinguishes incretin receptor triple agonists from single or double agonists, making unimolecular poly-pharmacology a useful tool for treating obesity's multiple pathways [296]. New triple-acting hybrid peptides show potential as obesity therapies in knock-out mice [297]. In all five clinical trials (SURPASS trials), Tirzepatide was the only medication to lower body weight (5.4–11.7 kg) in T2D when given weekly at doses of 5–15 mg [298]. Karagiannis et al. [299] found that Tirzepatide reduced weight better than basal insulin, placebo, and GLP-1RAs, with dosage enhancing the effect.
The GLP-1RAs assist T2D lose weight, control blood sugar, and improve cardiovascular health over time. As dose-dependent gastrointestinal symptoms limit efficacy, GLP-1 and GCG drugs that target alternative pathways may increase the therapeutic index [2].
9. Future directions and challenges
Numerous uncharted territories await investigation into GLP-1 in the years to come. Improving our understanding of the complex interplay between GLP-1RAs and their miRNA and other molecular targets is of the utmost importance. Targeted medicines with few adverse effects may become a reality once these pathways are understood. Envision GLP-1RAs developed to improve cancer treatment or tackle the underlying causes of Alzheimer's disease. In addition, GLP-1-specific tailored treatment is just around the corner. With the knowledge of miRNA profiles, we can personalize treatment plans to ensure that each patient gets the most out of it with the least amount of danger.
In addition, it is critical to address concerns about the safety and possible negative effects of GLP-1RAs. To provide a strong safety profile, long-term research is necessary. But studies aren't confined to the here and now. Discovering new uses that go beyond well-known goals, like aging healthily or avoiding chronic diseases, has tremendous potential. Do you think GLP-1RAs have the potential to delay or perhaps reverse the onset of diabetes in the future? To fully realize GLP-1's promise to transform healthcare for a wide range of chronic diseases, we need to optimize GLP-1RA design for individual diseases, create individualized treatment plans, and learn more about their mechanisms and long-term consequences.
10. Summary and conclusion
The GLP-1 has emerged as a therapeutic powerhouse, far exceeding its initial recognition as a gut hormone. This review has illuminated the diverse therapeutic potential of GLP-1RAs across a spectrum of chronic diseases. Their efficacy in managing T2D and obesity is well-established, with GLP-1RAs promoting glycemic control, weight loss, and even reducing cardiovascular complications in T2D patients.
However, the true magic of GLP-1RAs lies in their multifaceted effects. Emerging evidence suggests potential benefits in neurological disorders, and even a role in suppressing certain cancers. Unveiling the intricate molecular mechanisms underlying GLP-1RAs' action is crucial for maximizing their therapeutic potential. Understanding how they interact with pathways like ERK1/2, AMPK, and cAMP allows for targeted manipulation and potentially broader therapeutic applications.
Furthermore, the discovery of miRNAs as modulators of GLP-1 signaling opens exciting avenues for future research. By elucidating how specific miRNAs influence GLP-1 receptor function, we can pave the way for even more precise therapeutic strategies. Deciphering the interplay between GLP-1, miRNAs, and other regulatory pathways holds immense potential for personalized medicine, tailoring treatment to individual patient needs.
The future of GLP-1RAs is brimming with promise. As research delves deeper into their mechanisms and explores the potential of natural GLP-1 modulators, we can anticipate significant advancements. GLP-1RAs have the potential to revolutionize the management of various chronic diseases, ultimately improving health outcomes for a wider population.
CRediT authorship contribution statement
Ahmed I. Abulsoud: Supervision, Project administration, Investigation. Shereen Saeid Elshaer: Supervision, Project administration, Investigation. Toka Saber: Writing – original draft, Methodology, Investigation. Shahd Khaled: Writing – original draft, Methodology, Investigation. Mariam Hossam: Writing – original draft, Methodology, Investigation. Razan Ahmed: Writing – original draft, Methodology, Investigation. Nourhan M. Abdelmaksoud: Supervision, Project administration, Investigation. Aya Khaled: Writing – original draft, Methodology, Investigation. Rehab Abdelhamid: Writing – review & editing, Validation, Investigation, Formal analysis, Conceptualization. Tohada M. AL Noshokaty: Supervision, Project administration, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Data availability
All data is presented in the article.
References
- 1.Rowlands J., et al. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front. Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J.-Y., et al. GLP−1 receptor agonists for the treatment of obesity: role as a promising approach. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1085799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafferty R.A., et al. Proglucagon-derived peptides as therapeutics. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.689678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjørne A.P., Modvig I.M., Holst J.J. The sensory mechanisms of nutrient-induced GLP-1 secretion. Metabolites. 2022;12(5):420. doi: 10.3390/metabo12050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan R., Tomas A., Rutter G.A. Effects on pancreatic Beta and other Islet cells of the glucose-dependent insulinotropic polypeptide. Peptides. 2020;125 doi: 10.1016/j.peptides.2019.170201. [DOI] [PubMed] [Google Scholar]
- 6.Hare K.J. Role of GLP-1 induced glucagon suppression in type 2 diabetes mellitus. Dan. Med Bull. 2010;57(9):B4181. [PubMed] [Google Scholar]
- 7.Wendt A., Eliasson L. Pancreatic alpha cells and glucagon secretion: novel functions and targets in glucose homeostasis. Curr. Opin. Pharmacol. 2022;63 doi: 10.1016/j.coph.2022.102199. [DOI] [PubMed] [Google Scholar]
- 8.Tack J., et al. The gastrointestinal tract in hunger and satiety signalling. UEG J. 2021;9(6):727–734. doi: 10.1002/ueg2.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller T.D., et al. Glucagon-like peptide 1 (GLP-1) Mol. Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iovino M., et al. Neuroendocrine modulation of food intake and eating behavior. Endocr. Metab. Immune Disord. Drug Targets. 2022;22(13):1252–1262. doi: 10.2174/1871530322666220127114326. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg R.M., et al. Management of type 2 diabetes, obesity, or nonalcoholic steatohepatitis with high-dose GLP-1 receptor agonists and GLP-1 receptor-based co-agonists. Obes. Rev. 2024;25(3) doi: 10.1111/obr.13663. [DOI] [PubMed] [Google Scholar]
- 12.Brunton S.A., Wysham C.H. GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. Postgrad. Med. 2020;132(sup2):3–14. doi: 10.1080/00325481.2020.1798099. [DOI] [PubMed] [Google Scholar]
- 13.Giannakogeorgou A., Roden M. Role of lifestyle and glucagon-like peptide-1 receptor agonists for weight loss in obesity, type 2 diabetes and steatotic liver diseases. Aliment. Pharmacol. Ther. 2024;59:S52–S75. doi: 10.1111/apt.17848. [DOI] [PubMed] [Google Scholar]
- 14.Engin, A.B. and A. Engin, Protein kinases signaling in pancreatic beta-cells death and type 2 diabetes. Protein Kinase-mediated Decisions Between Life and Death, 2021: p. 195-227. [DOI] [PubMed]
- 15.Ma X., et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021;17(8):2050. doi: 10.7150/ijbs.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomas A., Jones B., Leech C. New insights into beta-cell GLP-1 receptor and cAMP signaling. J. Mol. Biol. 2020;432(5):1347–1366. doi: 10.1016/j.jmb.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Xiao J., et al. Control of human pancreatic beta cell kinome by glucagon-like peptide-1 receptor biased agonism. Diabetes, Obes. Metab. 2023;25(8):2105–2119. doi: 10.1111/dom.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X., et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front. Endocrinol. 2021;12:1040. doi: 10.3389/fendo.2021.721135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong G., et al. Effects of GLP-1 receptor agonists on biological behavior of colorectal cancer cells by regulating PI3K/AKT/mTOR signaling pathway. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radbakhsh S., et al. Incretins and microRNAs: interactions and physiological relevance. Pharmacol. Res. 2020;153 doi: 10.1016/j.phrs.2020.104662. [DOI] [PubMed] [Google Scholar]
- 21.Su J., et al. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int. J. Mol. Sci. 2023;24(17):13381. doi: 10.3390/ijms241713381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winiarska A., et al. Inflammation and oxidative stress in diabetic kidney disease: the targets for SGLT2 inhibitors and GLP-1 receptor agonists. Int. J. Mol. Sci. 2021;22(19):10822. doi: 10.3390/ijms221910822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavda V.P., et al. Unlocking longevity with GLP-1: a key to turn back the clock? Maturitas. 2024 doi: 10.1016/j.maturitas.2024.108028. [DOI] [PubMed] [Google Scholar]
- 24.Cantini G., et al. Hormonal Signaling in Biology and Medicine. Elsevier; 2020. Intestinal hormones; pp. 361–381. [Google Scholar]
- 25.Linderoth L., et al. GLP-1 receptor agonists for the treatment of type 2 diabetes and obesity. Success. Drug Discov. 2021:87–110. [Google Scholar]
- 26.Baggio L.L., et al. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 27.Kieffer T.J., Francis Habener J. The glucagon-like peptides. Endocr. Rev. 1999;20(6):876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 28.Cho Y.M., Fujita Y., Kieffer T.J. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu. Rev. Physiol. 2014;76:535–559. doi: 10.1146/annurev-physiol-021113-170315. [DOI] [PubMed] [Google Scholar]
- 29.Muskiet M.H., et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 2017;13(10):605–628. doi: 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 30.Nauck M.A., et al. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol. Metab. 2021;46 doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojtasińska A., et al. New insights into cardiovascular diseases treatment based on molecular targets. Int. J. Mol. Sci. 2023;24(23):16735. doi: 10.3390/ijms242316735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Campbell J.E., Newgard C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021;22(2):142–158. doi: 10.1038/s41580-020-00317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman, S.S., Gut hormone regulation of hepatic and intestinal lipoprotein production. 2023, University of Toronto (Canada).
- 35.Morais T., et al. Dysglycemia Shapes Visceral Adipose Tissue’s Response to GIP, GLP-1 and Glucagon in Individuals with Obesity. Metabolites. 2023;13(5):587. doi: 10.3390/metabo13050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monti G., et al. GLP-1 Receptor Agonists in Neurodegeneration: Neurovascular Unit in the Spotlight. Cells. 2022;11(13):2023. doi: 10.3390/cells11132023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nauck M.A., et al. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes, Obes. Metab. 2021;23(S3):5–29. doi: 10.1111/dom.14496. [DOI] [PubMed] [Google Scholar]
- 38.Rowlands J., et al. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lake D., Corrêa S.A., Müller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell. Mol. Life Sci. 2016;73(23):4397–4413. doi: 10.1007/s00018-016-2297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villavicencio A., et al. The effect of overweight and obesity on proliferation and activation of AKT and ERK in human endometria. Gynecol. Oncol. 2010;117(1):96–102. doi: 10.1016/j.ygyno.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Xu Z., et al. The role of ERK1/2 in the development of diabetic cardiomyopathy. Int. J. Mol. Sci. 2016;17(12):2001. doi: 10.3390/ijms17122001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan A.S., et al. ERK1 and ERK2 activation modulates diet-induced obesity in mice. Biochimie. 2017;137:78–87. doi: 10.1016/j.biochi.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Cai X., et al. GLP-1 treatment improves diabetic retinopathy by alleviating autophagy through GLP-1R-ERK1/2-HDAC6 signaling pathway. Int. J. Med. Sci. 2017;14(12):1203. doi: 10.7150/ijms.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma L., et al. Sennoside a induces GLP-1 secretion through activation of the ERK1/2 pathway in l-cells. Diabetes, Metab. Syndr. Obes. 2020:1407–1415. doi: 10.2147/DMSO.S247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang E., et al. Ezetimibe stimulates intestinal glucagon-like peptide 1 secretion via the MEK/ERK pathway rather than dipeptidyl peptidase 4 inhibition. Metabolism. 2015;64(5):633–641. doi: 10.1016/j.metabol.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Shi L., et al. Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc. Diabetol. 2015;14:1–13. doi: 10.1186/s12933-015-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshino Y., et al. Glucagon-like peptide-1 protects the murine hippocampus against stressors via Akt and ERK1/2 signaling. Biochem. Biophys. Res. Commun. 2015;458(2):274–279. doi: 10.1016/j.bbrc.2015.01.098. [DOI] [PubMed] [Google Scholar]
- 48.Kang G., et al. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J. Physiol. 2006;573(3):595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Britsch S., et al. Glucagon-like peptide-1 modulates Ca2+ current but not K+ ATP current in intact mouse pancreatic B-cells. Biochem. Biophys. Res. Commun. 1995;207(1):33–39. doi: 10.1006/bbrc.1995.1149. [DOI] [PubMed] [Google Scholar]
- 50.Smith N.K., et al. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019;128:94–105. doi: 10.1016/j.neuint.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doyle M.E., Egan J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007;113(3):546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meloni A., et al. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes, Obes. Metab. 2013;15(1):15–27. doi: 10.1111/j.1463-1326.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meloni A.R., et al. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes. Metab. 2013;15(1):15–27. doi: 10.1111/j.1463-1326.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., et al. Liraglutide enhances glucose transporter 4 translocation via regulation of AMP-activated protein kinase signaling pathways in mouse skeletal muscle cells. Metabolism. 2014;63(8):1022–1030. doi: 10.1016/j.metabol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Gromada J., Holst J.J., Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflügers Arch. 1998;435:583–594. doi: 10.1007/s004240050558. [DOI] [PubMed] [Google Scholar]
- 56.Sharma D., et al. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018;108:952–962. doi: 10.1016/j.biopha.2018.08.088. [DOI] [PubMed] [Google Scholar]
- 57.Zhou M., et al. The anti-diabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcinogenesis through cAMP–PKA–EGFR–STAT3 axis. Oncogene. 2017;36(29):4135–4149. doi: 10.1038/onc.2017.38. [DOI] [PubMed] [Google Scholar]
- 58.Kwon G., et al. cAMP dose-dependently prevents palmitate-induced apoptosis by both protein kinase A-and cAMP-guanine nucleotide exchange factor-dependent pathways in β-cells. J. Biol. Chem. 2004;279(10):8938–8945. doi: 10.1074/jbc.M310330200. [DOI] [PubMed] [Google Scholar]
- 59.Hosaka T., et al. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun. 2009;390(3):613–618. doi: 10.1016/j.bbrc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.H., et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes care. 2013;36(10):3276–3282. doi: 10.2337/dc13-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quoyer J., et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J. Biol. Chem. 2010;285(3):1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell F.M., Hardie D.G. AMP-Activated Protein Kinase: Do We Need Activators or Inhibitors to Treat or Prevent Cancer? Int J. Mol. Sci. 2020;22(1) doi: 10.3390/ijms22010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni Y., et al. Therapeutic inhibition of miR-802 protects against obesity through AMPK-mediated regulation of hepatic lipid metabolism. Theranostics. 2021;11(3):1079–1099. doi: 10.7150/thno.49354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Entezari M., et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharm. 2022;146 doi: 10.1016/j.biopha.2021.112563. [DOI] [PubMed] [Google Scholar]
- 65.Coughlan K.A., et al. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes, Metab. Syndr. Obes. 2014;7:241–253. doi: 10.2147/DMSO.S43731. (null) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andreozzi F., et al. The GLP-1 receptor agonists exenatide and liraglutide activate Glucose transport by an AMPK-dependent mechanism. J. Transl. Med. 2016;14(1):229. doi: 10.1186/s12967-016-0985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang B., et al. Liraglutide alleviates myocardial ischemia-reperfusion injury in diabetic mice. Mol. Cell. Endocrinol. 2023;572 doi: 10.1016/j.mce.2023.111954. [DOI] [PubMed] [Google Scholar]
- 69.Mazzieri A., et al. GLP-1 RAs and SGLT2i: two antidiabetic agents associated with immune and inflammation modulatory properties through the common AMPK pathway. Front Immunol. 2023;14:1163288. doi: 10.3389/fimmu.2023.1163288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H., et al. Liraglutide via Activation of AMP-Activated Protein Kinase-Hypoxia Inducible Factor-1α-Heme Oxygenase-1 Signaling Promotes Wound Healing by Preventing Endothelial Dysfunction in Diabetic Mice. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.660263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo Z., et al. Effect of exenatide on the cardiac expression of adiponectin receptor 1 and NADPH oxidase subunits and heart function in streptozotocin-induced diabetic rats. Diabetol. Metab. Syndr. 2014;6(1):29. doi: 10.1186/1758-5996-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moellmann J., et al. Glucagon-Like Peptide 1 and Its Cleavage Products Are Renoprotective in Murine Diabetic Nephropathy. Diabetes. 2018;67(11):2410–2419. doi: 10.2337/db17-1212. [DOI] [PubMed] [Google Scholar]
- 73.Xu F., et al. Short-term GLP-1 receptor agonist exenatide ameliorates intramyocellular lipid deposition without weight loss in ob/ob mice. Int. J. Obes. 2020;44(4):937–947. doi: 10.1038/s41366-019-0513-y. [DOI] [PubMed] [Google Scholar]
- 74.Kim E.K., Choi E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Et. Biophys. Acta (BBA)-Mol. Basis Dis. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Ma J., et al. GLP‑1R agonists ameliorate peripheral nerve dysfunction and inflammation via p38 MAPK/NF‑κB signaling pathways in streptozotocin‑induced diabetic rats. Int. J. Mol. Med. 2018;41(5):2977–2985. doi: 10.3892/ijmm.2018.3509. [DOI] [PubMed] [Google Scholar]
- 76.Chen J., et al. GLP-1 receptor agonist as a modulator of innate immunity. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.997578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye Y., et al. Protective effects of liraglutide on glomerular podocytes in obese mice by inhibiting the inflammatory factor TNF-α-mediated NF-κB and MAPK pathway. Obes. Res. Clin. Pract. 2019;13(4):385–390. doi: 10.1016/j.orcp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Peng L., et al. The protective effect of beraprost sodium on diabetic nephropathy by inhibiting inflammation and p38 MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic rats. Int. J. Endocrinol. 2016;2016 doi: 10.1155/2016/1690474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barros J., da Silva Santos R., da Silva Reis A.A. Implication of the MAPK signalling pathway in the pathogenesis of diabetic nephropathy. Diabetes. 2019;7(1):107–114. [Google Scholar]
- 80.Li Z., et al. Liraglutide, a glucagon-like peptide-1 receptor agonist, suppresses osteoclastogenesis through the inhibition of NF-κB and MAPK pathways via GLP-1R. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110523. [DOI] [PubMed] [Google Scholar]
- 81.Pujadas G., et al. The pivotal role of high glucose-induced overexpression of PKCβ in the appearance of glucagon-like peptide-1 resistance in endothelial cells. Endocrine. 2016;54:396–410. doi: 10.1007/s12020-015-0799-z. [DOI] [PubMed] [Google Scholar]
- 82.Shigeto M., et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J. Clin. Investig. 2015;125(12):4714–4728. doi: 10.1172/JCI81975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan X., et al. Essential role of high glucose-induced overexpression of PKCβ and PKCδ In GLP-1 resistance In rodent cardiomyocytes. Diabetes, Metab. Syndr. Obes.: Targets Ther. 2019:2289–2302. doi: 10.2147/DMSO.S215789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L., et al. Protein kinase C pathway mediates the protective effects of glucagon-like peptide-1 on the apoptosis of islet β-cells. Mol. Med. Rep. 2015;12(5):7589–7594. doi: 10.3892/mmr.2015.4355. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki Y., et al. Glucagon-like peptide 1 activates protein kinase C through Ca2+ -dependent activation of phospholipase C in insulin-secreting cells. J. Biol. Chem. 2006;281(39):28499–28507. doi: 10.1074/jbc.M604291200. [DOI] [PubMed] [Google Scholar]
- 86.Youssef N., et al. Reno-Protective Effect of GLP-1 Receptor Agonists in Type1 Diabetes: Dual Action on TRPC6 and NADPH Oxidases. Biomedicines. 2021;9(10):1360. doi: 10.3390/biomedicines9101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Q., et al. Semaglutide inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis through activating PKG/PKCε/ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2023;647:1–8. doi: 10.1016/j.bbrc.2023.01.049. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z., et al. GLP-1 inhibits PKCβ2 phosphorylation to improve the osteogenic differentiation potential of hPDLSCs in the AGE microenvironment. J. Diabetes its Complicat. 2020;34(3) doi: 10.1016/j.jdiacomp.2019.107495. [DOI] [PubMed] [Google Scholar]
- 89.Yusta B., et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64(7):2537–2549. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 90.Sandoval D.A. and D.A. D′Alessio, Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015;95(2):513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 91.Hope D.C., Vincent M.L., Tan T.M. Striking the balance: GLP-1/glucagon co-agonism as a treatment strategy for obesity. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.735019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lazzaroni E., et al. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol. Res. 2021;171 doi: 10.1016/j.phrs.2021.105782. [DOI] [PubMed] [Google Scholar]
- 93.Ja’arah D., et al. Role of Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in Hypoglycemia. Clin. Med. Insight.: Endocrinol. Diabetes. 2021;14 doi: 10.1177/11795514211051697. p. 11795514211051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Drucker D.J., Habener J.F., Holst J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Investig. 2017;127(12):4217–4227. doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramracheya R., et al. GLP-1 suppresses glucagon secretion in human pancreatic alpha-cells by inhibition of P/Q-type Ca2+ channels. Physiol. Rep. 2018;6(17) doi: 10.14814/phy2.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodrigues T., et al. GLP-1 improves adipose tissue glyoxalase activity and capillarization improving insulin sensitivity in type 2 diabetes. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105198. [DOI] [PubMed] [Google Scholar]
- 97.Tran K.L., et al. Overview of Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Patients with Type 2 Diabetes. Am. Health Drug Benefits. 2017;10(4):178–188. [PMC free article] [PubMed] [Google Scholar]
- 98.Jepsen M.M., Christensen M.B. Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expert Opin. Emerg. Drugs. 2021;26(3):231–243. doi: 10.1080/14728214.2021.1947240. [DOI] [PubMed] [Google Scholar]
- 99.Graaf C., et al. Glucagon-like peptide-1 and its class BG protein–coupled receptors: a long march to therapeutic successes. Pharmacol. Rev. 2016;68(4):954–1013. doi: 10.1124/pr.115.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerstein H.C., et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 101.Greco E.V., et al. GLP-1 Receptor Agonists and Kidney Protection. Medicina. 2019;55(6):233. doi: 10.3390/medicina55060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li X., et al. Effect of glucagon-like peptide 1 receptor agonists on the renal protection in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2022;48(5) doi: 10.1016/j.diabet.2022.101366. [DOI] [PubMed] [Google Scholar]
- 103.Mann J.F., et al. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1–7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(11):880–893. doi: 10.1016/S2213-8587(20)30313-2. [DOI] [PubMed] [Google Scholar]
- 104.von Scholten B.J., et al. The potential of GLP-1 receptor agonists in type 2 diabetes and chronic kidney disease: from randomised trials to clinical practice. Ther. Adv. Endocrinol. Metab. 2022;13 doi: 10.1177/20420188221112490. 20420188221112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mosterd C.M., Bjornstad P., van Raalte D.H. Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J. Nephrol. 2020;33:965–975. doi: 10.1007/s40620-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaur P., et al. Clinical experience with Liraglutide in 196 patients with type 2 diabetes from a tertiary care center in India. Indian J. Endocrinol. Metab. 2014;18(1):77. doi: 10.4103/2230-8210.126572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sorli C., et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–260. doi: 10.1016/S2213-8587(17)30013-X. [DOI] [PubMed] [Google Scholar]
- 108.Rodbard H.W., et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care. 2019;42(12):2272–2281. doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 109.Pratley R.E., et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 110.Bader G., Geransar P., Schweizer A. Vildagliptin more effectively achieves a composite endpoint of HbA1c< 7.0% without hypoglycaemia and weight gain compared with glimepiride after 2 years of treatment. Diabetes Res. Clin. Pract. 2013;100(3):e78–e81. doi: 10.1016/j.diabres.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 111.De Lorenzo A., et al. Obesity: A preventable, treatable, but relapsing disease. Nutrition. 2020;71 doi: 10.1016/j.nut.2019.110615. [DOI] [PubMed] [Google Scholar]
- 112.Spencer L. University of Derby; United Kingdom: 2023. Investigation of the Metabolic Effects of Liraglutide on Patients with Overweight and Obesity. [Google Scholar]
- 113.Lian K., et al. Emerging therapeutic landscape: Incretin agonists in chronic kidney disease management. Life Sci. 2024 doi: 10.1016/j.lfs.2024.122801. [DOI] [PubMed] [Google Scholar]
- 114.Wang J.-Y., et al. GLP− 1 receptor agonists for the treatment of obesity: Role as a promising approach. Front. Endocrinol. 2023;14:1085799. doi: 10.3389/fendo.2023.1085799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ranganath L.R., et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38(6):916–919. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.RANGANATH L., et al. Inhibition of carbohydrate-mediated glucagon-like peptide-I (7–36)amide secretion by circulating non-esterified fatty acids. Clin. Sci. 1999;96(4):335–342. doi: 10.1042/cs0960335. [DOI] [PubMed] [Google Scholar]
- 117.Vrang N., Larsen P.J. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: Role of peripherally secreted and centrally produced peptides. Prog. Neurobiol. 2010;92(3):442–462. doi: 10.1016/j.pneurobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 118.Smits M.M., et al. Gastrointestinal actions of glucagon-like peptide-1-based therapies: glycaemic control beyond the pancreas. Diabetes, Obes. Metab. 2016;18(3):224–235. doi: 10.1111/dom.12593. [DOI] [PubMed] [Google Scholar]
- 119.Kanoski S.E., Hayes M.R., Skibicka K.P. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am. J. Physiol. -Regul., Integr. Comp. Physiol. 2016;310(10):R885–R895. doi: 10.1152/ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanoski S.E., et al. Peripheral and Central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1 Receptor Agonists, Liraglutide and Exendin-4. Endocrinology. 2011;152(8):3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holst J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 122.Beiroa D., et al. GLP-1 Agonism Stimulates Brown Adipose Tissue Thermogenesis and Browning Through Hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 123.Burmeister M.A., et al. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am. J. Physiol. -Endocrinol. Metab. 2013;304(7):E677–E685. doi: 10.1152/ajpendo.00446.2012. [DOI] [PubMed] [Google Scholar]
- 124.Kooijman S., et al. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia. 2015;58(11):2637–2646. doi: 10.1007/s00125-015-3727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu F., et al. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia. 2016;59(5):1059–1069. doi: 10.1007/s00125-016-3896-5. [DOI] [PubMed] [Google Scholar]
- 126.Cantini G., Mannucci E., Luconi M. Perspectives in GLP-1 research: new targets, new receptors. Trends Endocrinol. Metab. 2016;27(6):427–438. doi: 10.1016/j.tem.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 127.Sélley E., et al. Vasodilator effect of glucagon: receptorial crosstalk among glucagon, GLP-1, and receptor for glucagon and GLP-1. Horm. Metab. Res. 2016;48(07):476–483. doi: 10.1055/s-0042-101794. [DOI] [PubMed] [Google Scholar]
- 128.Bakbak E., et al. Lessons from bariatric surgery: can increased GLP-1 enhance vascular repair during cardiometabolic-based chronic disease? Rev. Endocr. Metab. Disord. 2021:1–18. doi: 10.1007/s11154-021-09669-7. [DOI] [PubMed] [Google Scholar]
- 129.Hirsch I.B. The future of the GLP-1 receptor agonists. Jama. 2019;321(15):1457–1458. doi: 10.1001/jama.2019.2941. [DOI] [PubMed] [Google Scholar]
- 130.Wen S., et al. An overview of similarities and differences in metabolic actions and effects of central nervous system between glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium glucose co-transporter-2 inhibitors (SGLT-2is) Diabetes, Metab. Syndr. Obes. 2021:2955–2972. doi: 10.2147/DMSO.S312527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang L., Zhang W., Tian X. The pleiotropic of GLP-1/GLP-1R axis in central nervous system diseases. Int. J. Neurosci. 2023;133(5):473–491. doi: 10.1080/00207454.2021.1924707. [DOI] [PubMed] [Google Scholar]
- 132.He W., et al. Role of liraglutide in brain repair promotion through Sirt1-mediated mitochondrial improvement in stroke. J. Cell. Physiol. 2020;235(3):2986–3001. doi: 10.1002/jcp.29204. [DOI] [PubMed] [Google Scholar]
- 133.Basalay M.V., Davidson S.M., Yellon D.M. Neuroprotection in Rats Following Ischaemia-Reperfusion Injury by GLP-1 Analogues—Liraglutide and Semaglutide. Cardiovasc. Drugs Ther. 2019;33(6):661–667. doi: 10.1007/s10557-019-06915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grieco M., et al. Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou Y., et al. Exendin-4 improves behaviorial deficits via GLP-1/GLP-1R signaling following partial hepatectomy. Brain Res. 2019;1706:116–124. doi: 10.1016/j.brainres.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L.-Q., et al. GLP-1R activation ameliorated novel-object recognition memory dysfunction via regulating hippocampal AMPK/NF-κB pathway in neuropathic pain mice. Neurobiol. Learn. Mem. 2021;182 doi: 10.1016/j.nlm.2021.107463. [DOI] [PubMed] [Google Scholar]
- 137.Nauck M.A., Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care. 2013;36(2):S245–S252. doi: 10.2337/dcS13-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elashoff M., et al. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1–Based Therapies. Gastroenterology. 2011;141(1):150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nachnani J.S., et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 2010;53(1):153–159. doi: 10.1007/s00125-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 140.Cao C., Yang S., Zhou Z. GLP-1 receptor agonists and risk of cancer in type 2 diabetes: an updated meta-analysis of randomized controlled trials. Endocrine. 2019;66(2):157–165. doi: 10.1007/s12020-019-02055-z. [DOI] [PubMed] [Google Scholar]
- 141.Pinto L.C., et al. Glucagon-like peptide-1 receptor agonists and pancreatic cancer: a meta-analysis with trial sequential analysis. Sci. Rep. 2019;9(1):2375. doi: 10.1038/s41598-019-38956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wicki A., et al. [Lys40(Ahx-DTPA-111In)NH2]-Exendin-4 Is a Highly Efficient Radiotherapeutic for Glucagon-Like Peptide-1 Receptor–Targeted Therapy for Insulinoma. Clin. Cancer Res. 2007;13(12):3696–3705. doi: 10.1158/1078-0432.CCR-06-2965. [DOI] [PubMed] [Google Scholar]
- 143.Nomiyama T., et al. Exendin-4, a GLP-1 Receptor Agonist, Attenuates Prostate Cancer Growth. Diabetes. 2014;63(11):3891–3905. doi: 10.2337/db13-1169. [DOI] [PubMed] [Google Scholar]
- 144.Iwaya C., et al. Exendin-4, a Glucagonlike Peptide-1 Receptor Agonist, Attenuates Breast Cancer Growth by Inhibiting NF-κB Activation. Endocrinology. 2017;158(12):4218–4232. doi: 10.1210/en.2017-00461. [DOI] [PubMed] [Google Scholar]
- 145.Ligumsky H., et al. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res. Treat. 2012;132(2):449–461. doi: 10.1007/s10549-011-1585-0. [DOI] [PubMed] [Google Scholar]
- 146.Nomiyama T., Yanase T. GLP-1 receptor agonist as treatment for cancer as well as diabetes: beyond blood glucose control. Expert Rev. Endocrinol. Metab. 2016;11(4):357–364. doi: 10.1080/17446651.2016.1191349. [DOI] [PubMed] [Google Scholar]
- 147.Zhao H., et al. Activation of glucagon-like peptide-1 receptor inhibits tumourigenicity and metastasis of human pancreatic cancer cells via PI3K/Akt pathway. Diabetes, Obes. Metab. 2014;16(9):850–860. doi: 10.1111/dom.12291. [DOI] [PubMed] [Google Scholar]
- 148.Bendotti G., et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022;182 doi: 10.1016/j.phrs.2022.106320. [DOI] [PubMed] [Google Scholar]
- 149.Currie C.J., Poole C.D., Gale E. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 150.Bezin J., et al. GLP-1 Receptor Agonists and the Risk of Thyroid Cancer. Diabetes Care. 2022;46(2):384–390. doi: 10.2337/dc22-1148. [DOI] [PubMed] [Google Scholar]
- 151.Cases A.I., et al. Significance of expression of glucagon-like peptide 1 receptor in pancreatic cancer. Oncol. Rep. 2015;34(4):1717–1725. doi: 10.3892/or.2015.4138. [DOI] [PubMed] [Google Scholar]
- 152.Stein M.S., et al. The GLP-1 receptor is expressed in vivo by human metastatic prostate cancer. Endocr. Oncol. 2024;4(1) doi: 10.1530/EO-23-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shigeoka T., et al. Activation of overexpressed glucagon-like peptide-1 receptor attenuates prostate cancer growth by inhibiting cell cycle progression. J. Diabetes Investig. 2020;11(5):1137–1149. doi: 10.1111/jdi.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Piccoli G.F., et al. Do GLP-1 receptor agonists increase the risk of breast cancer? A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021;106(3):912–921. doi: 10.1210/clinem/dgaa891. [DOI] [PubMed] [Google Scholar]
- 155.Zhang X., et al. GLP-1 receptor agonist liraglutide inhibits the proliferation and migration of thyroid cancer cells. Cell. Mol. Biol. 2023;69(14):221–225. doi: 10.14715/cmb/2023.69.14.37. [DOI] [PubMed] [Google Scholar]
- 156.O'Brien J., et al. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lai X., Wolkenhauer O., Vera J. Understanding microRNA-mediated gene regulatory networks through mathematical modelling. Nucleic Acids Res. 2016;44(13):6019–6035. doi: 10.1093/nar/gkw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Klinge C.M. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol. Cell Endocrinol. 2015;418(0 3):273–297. doi: 10.1016/j.mce.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Margaritis K., et al. Micro-RNA Implications in Type-1 Diabetes Mellitus: A Review of Literature. Int J. Mol. Sci. 2021;22(22) doi: 10.3390/ijms222212165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Grieco G.E., et al. Circulating microRNAs as clinically useful biomarkers for Type 2 Diabetes Mellitus: miRNomics from bench to bedside. Transl. Res. 2022;247:137–157. doi: 10.1016/j.trsl.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 161.Formichi C., et al. Circulating microRNAs Signature for Predicting Response to GLP1-RA Therapy in Type 2 Diabetic Patients: A Pilot Study. Int J. Mol. Sci. 2021;22(17) doi: 10.3390/ijms22179454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nasser J.S., et al. The Role of MicroRNA, Long Non-Coding RNA and Circular RNA in the Pathogenesis of Polycystic Ovary Syndrome: A Literature Review. Int J. Mol. Sci. 2024;25(2) doi: 10.3390/ijms25020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miao C., et al. MicroRNAs in type 1 diabetes: new research progress and potential directions. Biochem Cell Biol. 2018;96(5):498–506. doi: 10.1139/bcb-2018-0027. [DOI] [PubMed] [Google Scholar]
- 164.He X., et al. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin. Transl. Med. 2021;11(6) doi: 10.1002/ctm2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mudhasani R., Imbalzano A.N., Jones S.N. An essential role for Dicer in adipocyte differentiation. J. Cell Biochem. 2010;110(4):812–816. doi: 10.1002/jcb.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Silveira A., et al. MicroRNAs in Obesity-Associated Disorders: The Role of Exercise Training. Obes. Facts. 2022;15(2):105–117. doi: 10.1159/000517849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matarese A., et al. miR-7 Regulates GLP-1-Mediated Insulin Release by Targeting β-Arrestin 1. 2020;9(7):1621. doi: 10.3390/cells9071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jo S., et al. miR-204 Controls Glucagon-Like Peptide 1 Receptor Expression and Agonist Function. Diabetes. 2017;67(2):256–264. doi: 10.2337/db17-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yao Y., et al. Glucagon-Like Peptide-1 Modulates Cholesterol Homeostasis by Suppressing the miR-19b-Induced Downregulation of ABCA1. Cell Physiol. Biochem. 2018;50(2):679–693. doi: 10.1159/000494235. [DOI] [PubMed] [Google Scholar]
- 170.Shang J., et al. Induction of miR-132 and miR-212 Expression by Glucagon-Like Peptide 1 (GLP-1) in Rodent and Human Pancreatic β-Cells. Mol. Endocrinol. 2015;29(9):1243–1253. doi: 10.1210/me.2014-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu J., et al. Glucagon-like peptide-1 (GLP-1) improved diabetic lung fibrosis via AMPK and microRNA-27a (miR-27a) Ann. Transl. Med. 2021;9(6):492. doi: 10.21037/atm-21-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pan W., et al. miR‑192 is upregulated in T1DM, regulates pancreatic β‑cell development and inhibits insulin secretion through suppressing GLP‑1 expression. Exp. Ther. Med. 2018;16(3):2717–2724. doi: 10.3892/etm.2018.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Y. Effect of intestinal microbiota imbalance associated with chronic hepatitis B virus infection on the expression of microRNA‑192 and GLP‑1. Mol. Med Rep. 2021;24(3):662. doi: 10.3892/mmr.2021.12301. [DOI] [PubMed] [Google Scholar]
- 174.Wang J., et al. Berberine activates the β-catenin/TCF4 signaling pathway by down-regulating miR-106b to promote GLP-1 production by intestinal L cells. Eur. J. Pharmacol. 2021;911 doi: 10.1016/j.ejphar.2021.174482. [DOI] [PubMed] [Google Scholar]
- 175.Yao Y., et al. Glucagon-like peptide-1 contributes to increases ABCA1 expression by downregulating miR-758 to regulate cholesterol homeostasis. Biochem. Biophys. Res. Commun. 2018;497(2):652–658. doi: 10.1016/j.bbrc.2018.02.126. [DOI] [PubMed] [Google Scholar]
- 176.Liu, Y., D. Nie, and X. Lou, The cardiovascular benefits of glucagon-like peptide-1 receptor agonists as novel diabetes drugs are mediated via the suppression of miR-203a-3p and miR-429 expression. 2023. [DOI] [PubMed]
- 177.Han Y.-b, et al. MicroRNA-34a contributes to the protective effects of glucagon-like peptide-1 against lipotoxicity in INS-1 cells. Chin. Med. J. 2012;125(23) [PubMed] [Google Scholar]
- 178.Kreymann B., et al. GLUCAGON-LIKE PEPTIDE-1 7-36: A PHYSIOLOGICAL INCRETIN IN MAN. Lancet. 1987;330(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 179.Li P., et al. A novel epigenetic mechanism of FXR inhibiting GLP-1 secretion via miR-33 and its downstream targets. Biochem. Biophys. Res. Commun. 2019;517(4):629–635. doi: 10.1016/j.bbrc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 180.Coskun Z.M., Beydogan A.B., Bolkent S. Changes in the expression levels of CB1 and GLP-1R mRNAs and microRNAs 33a and 122 in the liver of type 2 diabetic rats treated with ghrelin. J. Biochem. Mol. Toxicol. 2019;33(10) doi: 10.1002/jbt.22388. [DOI] [PubMed] [Google Scholar]
- 181.Wang C., et al. GLP-1 contributes to increases in PGC-1α expression by downregulating miR-23a to reduce apoptosis. Biochem. Biophys. Res. Commun. 2015;466(1):33–39. doi: 10.1016/j.bbrc.2015.08.092. [DOI] [PubMed] [Google Scholar]
- 182.Wang Q., Du J., Ma R. White adipocyte-derived exosomal miR-23b inhibits thermogenesis by targeting Elf4 to regulate GLP-1R transcription. Naunyn Schmiede Arch. Pharm. 2024 doi: 10.1007/s00210-024-02984-1. [DOI] [PubMed] [Google Scholar]
- 183.Asaad G.F., et al. Exploring cutting-edge approaches in diabetes care: from nanotechnology to personalized therapeutics. Naunyn-Schmiede 'S. Arch. Pharmacol. 2024 doi: 10.1007/s00210-024-03532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arte P.A., et al. Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach. Hum. Cell. 2024;37(1):54–84. doi: 10.1007/s13577-023-01007-0. [DOI] [PubMed] [Google Scholar]
- 185.Lafferty R.A., Flatt P.R., Irwin N. GLP-1/GIP analogs: potential impact in the landscape of obesity pharmacotherapy. Expert Opin. Pharmacother. 2023;24(5):587–597. doi: 10.1080/14656566.2023.2192865. [DOI] [PubMed] [Google Scholar]
- 186.Borah A.K., Ahmed S.A., Borah J.C. Phytomedicine as a source of SGLT2 inhibitors, GLP-1 secretagogues and DPP-IV inhibitors for mitigation of diabetic nephropathy. Phytomedicine. 2022;2(2) [Google Scholar]
- 187.Nooreen Z., et al. An insight of naturally occurring phytoconstituents and novel approaches towards the treatment of diabetes. Curr. Diabetes Rev. 2024;20(3):136–148. doi: 10.2174/1573399820666230829094724. [DOI] [PubMed] [Google Scholar]
- 188.Rahman M.M., et al. The multifunctional role of herbal products in the management of diabetes and obesity: a comprehensive review. Molecules. 2022;27(5):1713. doi: 10.3390/molecules27051713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cortez-Navarrete M., Pérez-Rubio K.G. and M.d.J. Escobedo-Gutiérrez, Role of fenugreek, cinnamon, Curcuma longa, berberine and Momordica charantia in type 2 diabetes mellitus treatment: a review. Pharmaceuticals. 2023;16(4):515. doi: 10.3390/ph16040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Blahova J., et al. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals. 2021;14(8):806. doi: 10.3390/ph14080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yaribeygi, H., et al., Boosting GLP-1 by natural products. Natural Products and Human Diseases: Pharmacology, Molecular Targets, and Therapeutic Benefits, 2021: p. 513-522. [DOI] [PubMed]
- 192.Khunti K., et al. Associations between second-line glucose-lowering combination therapies with metformin and HbA1c, body weight, quality of life, hypoglycaemic events and glucose-lowering treatment intensification: the DISCOVER study. Diabetes, Obes. Metab. 2021;23(8):1823–1833. doi: 10.1111/dom.14400. [DOI] [PubMed] [Google Scholar]
- 193.Hu Y., et al. Cost-effectiveness analysis of 4 GLP-1RAs in the treatment of obesity in a US setting. Ann. Transl. Med. 2022;10(3) doi: 10.21037/atm-22-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mohan V., et al. Management of type 2 diabetes in developing countries: balancing optimal glycaemic control and outcomes with affordability and accessibility to treatment. Diabetes Ther. 2020;11:15–35. doi: 10.1007/s13300-019-00733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gilbert M.P., Pratley R.E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Abiola J.O., et al. Potential Role of Phytochemicals as Glucagon-like Peptide 1 Receptor (GLP-1R) Agonists in the Treatment of Diabetes Mellitus. Pharmaceuticals. 2024;17(6):736. doi: 10.3390/ph17060736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Holt R.I., et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes care. 2021;44(11):2589–2625. doi: 10.2337/dci21-0043. [DOI] [PubMed] [Google Scholar]
- 198.Nejati L., et al. The effect of berberine on lipid profile, liver enzymes, and fasting blood glucose in patients with non-alcoholic fatty liver disease (NAFLD): a randomized controlled trial. Med. J. Islam. Repub. Iran. 2022;36 doi: 10.47176/mjiri.36.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yu Y., et al. Berberine induces GLP-1 secretion through activation of bitter taste receptor pathways. Biochem. Pharmacol. 2015;97(2):173–177. doi: 10.1016/j.bcp.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 200.Nagasawa T., et al. Teadenol A in microbial fermented tea acts as a novel ligand on GPR120 to increase GLP-1 secretion. Food Funct. 2020;11(12):10534–10541. doi: 10.1039/d0fo02442b. [DOI] [PubMed] [Google Scholar]
- 201.Alli-Oluwafuyi A.-M., et al. Curcumin induces secretion of glucagon-like peptide-1 through an oxidation-dependent mechanism. Biochimie. 2019;165:250–257. doi: 10.1016/j.biochi.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bloomer R., et al. A Mixture of Glycine and Cinnamon Extract Improves Glucose Uptake and Imparts Favourable Metabolic Changes in Men and Women in Response to an Oral Glucose Load. Endocrinol. Metab. Syndr. 2018;7(289) 2161-1017. 1000289. [Google Scholar]
- 203.Yuan H., et al. Bioactive peptides of plant origin: distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022;13(6):3133–3158. doi: 10.1039/d1fo04077d. [DOI] [PubMed] [Google Scholar]
- 204.Li M., Wu Y., Ye L. The role of amino acids in endothelial biology and function. Cells. 2022;11(8):1372. doi: 10.3390/cells11081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jamali N., et al. Effect of cinnamon supplementation on blood pressure and anthropometric parameters in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Diabetes Metab. Syndr.: Clin. Res. Rev. 2020;14(2):119–125. doi: 10.1016/j.dsx.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 206.Silva M.L., et al. Cinnamon as a complementary therapeutic approach for dysglycemia and dyslipidemia control in type 2 diabetes mellitus and its molecular mechanism of action: A review. Nutrients. 2022;14(13):2773. doi: 10.3390/nu14132773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shang C., et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021;12(24):12194–12220. doi: 10.1039/d1fo01935j. [DOI] [PubMed] [Google Scholar]
- 208.Ziegenfuss T.N., et al. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J. Int. Soc. Sports Nutr. 2006;3(2):45. doi: 10.1186/1550-2783-3-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kato M., et al. Low-molecular fraction of wheat protein hydrolysate stimulates glucagon-like peptide-1 secretion in an enteroendocrine L cell line and improves glucose tolerance in rats. Nutr. Res. 2017;37:37–45. doi: 10.1016/j.nutres.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 210.Eelderink C., et al. Difference in postprandial GLP-1 response despite similar glucose kinetics after consumption of wheat breads with different particle size in healthy men. Eur. J. Nutr. 2017;56:1063–1076. doi: 10.1007/s00394-016-1156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Samad M.B., et al. 6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement. Altern. Med. 2017;17(1):1–13. doi: 10.1186/s12906-017-1903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Phuwamongkolwiwat P., et al. Fructooligosaccharide augments benefits of quercetin-3-O-β-glucoside on insulin sensitivity and plasma total cholesterol with promotion of flavonoid absorption in sucrose-fed rats. Eur. J. Nutr. 2014;53:457–468. doi: 10.1007/s00394-013-0546-2. [DOI] [PubMed] [Google Scholar]
- 213.Khan N., Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2018;11(1):39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rageh M.M., et al. Physico-chemical properties of curcumin nanoparticles and its efficacy against Ehrlich ascites carcinoma. Sci. Rep. 2023;13(1):20637. doi: 10.1038/s41598-023-47255-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jayaprakasha G.K., Rao L.J. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011;51(6):547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 216.Zhang Y.L., et al. Beinaglutide showed significant weight-loss benefit and effective glycaemic control for the treatment of type 2 diabetes in a real-world setting: a 3-month, multicentre, observational, retrospective, open-label study. Obes. Sci. Pract. 2019;5(4):366–375. doi: 10.1002/osp4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., et al. Effect of beinaglutide treatment on weight loss in Chinese patients with type 2 diabetes mellitus and overweight/obesity. Arch. Endocrinol. Metab. 2021;65(4):421–427. doi: 10.20945/2359-3997000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fang X., et al. Beinaglutide shows significantly beneficial effects in diabetes/obesity-induced nonalcoholic steatohepatitis in ob/ob mouse model. Life Sci. 2021;270 doi: 10.1016/j.lfs.2020.118966. [DOI] [PubMed] [Google Scholar]
- 219.Gao L., et al. Comparison of Beinaglutide Versus Metformin for Weight Loss in Overweight and Obese Non-diabetic Patients. Exp. Clin. Endocrinol. Diabetes. 2021;130(06):358–367. doi: 10.1055/a-1608-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang F., et al. Recombinant human GLP-1 beinaglutide regulates lipid metabolism of adipose tissues in diet-induced obese mice. iScience. 2021;24(12) doi: 10.1016/j.isci.2021.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Folli F., Guardado Mendoza R. Potential use of exenatide for the treatment of obesity. Expert Opin. Investig. Drugs. 2011;20(12):1717–1722. doi: 10.1517/13543784.2011.630660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Barnett A. Exenatide. Expert Opin. Pharmacother. 2007;8(15):2593–2608. doi: 10.1517/14656566.8.15.2593. [DOI] [PubMed] [Google Scholar]
- 223.Simonsen L., Holst J.J., Deacon C.F. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia. 2006;49(4):706–712. doi: 10.1007/s00125-005-0128-9. [DOI] [PubMed] [Google Scholar]
- 224.Nishi H., Higashihara T., Inagi R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients. 2019;11(7):1664. doi: 10.3390/nu11071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang Y., et al. Exenatide Attenuates Obesity-Induced Mitochondrial Dysfunction by Activating SIRT1 in Renal Tubular Cells. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.622737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Moretto T.J., et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug—naive patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel-group study. Clin. Ther. 2008;30(8):1448–1460. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Buse J.B., et al. Effects of Exenatide (Exendin-4) on Glycemic Control Over 30 Weeks in Sulfonylurea-Treated Patients With Type 2 Diabetes. Diabetes Care. 2004;27(11):2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 228.Apovian C.M., et al. Effects of Exenatide Combined with Lifestyle Modification in Patients with Type 2 Diabetes. Am. J. Med. 2010;123(5):468.e9–468.e17. doi: 10.1016/j.amjmed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 229.Rosenstock J., et al. Effects of Exenatide and Lifestyle Modification on Body Weight and Glucose Tolerance in Obese Subjects With and Without Pre-Diabetes. Diabetes Care. 2010;33(6):1173–1175. doi: 10.2337/dc09-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Elkind-Hirsch K., et al. Comparison of Single and Combined Treatment with Exenatide and Metformin on Menstrual Cyclicity in Overweight Women with Polycystic Ovary Syndrome. The. J. Clin. Endocrinol. Metab. 2008;93(7):2670–2678. doi: 10.1210/jc.2008-0115. [DOI] [PubMed] [Google Scholar]
- 231.Effect of Exenatide on Fat Deposition and a Metabolic Profile in Patients with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2011;9(1):31–34. doi: 10.1089/met.2010.0025. [DOI] [PubMed] [Google Scholar]
- 232.Iepsen E.W., Torekov S.S., Holst J.J. Therapies for inter-relating diabetes and obesity – GLP-1 and obesity. Expert Opin. Pharmacother. 2014;15(17):2487–2500. doi: 10.1517/14656566.2014.965678. [DOI] [PubMed] [Google Scholar]
- 233.Anderson S.L., Trujillo J.M. Lixisenatide in type 2 diabetes: latest evidence and clinical usefulness. Ther. Adv. Chronic Dis. 2016;7(1):4–17. doi: 10.1177/2040622315609312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Petersen A.B., Knop F.K., Christensen M. Lixisenatide for the treatment of type 2 diabetes. Drugs Today (Barc., Spain.: 1998) 2013;49(9):537–553. doi: 10.1358/dot.2013.49.9.2020940. [DOI] [PubMed] [Google Scholar]
- 235.Pfeffer M.A., et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 236.Fonseca V.A., et al. Efficacy and Safety of the Once-Daily GLP-1 Receptor Agonist Lixisenatide in Monotherapy: A randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono) Diabetes Care. 2012;35(6):1225–1231. doi: 10.2337/dc11-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McCarty D., Coleman M., Boland C.L. Lixisenatide: A New Daily GLP-1 Agonist for Type 2 Diabetes Management. Ann. Pharmacother. 2017;51(5):401–409. doi: 10.1177/1060028017689878. [DOI] [PubMed] [Google Scholar]
- 238.Forst T., Pfützner A. Pharmacological profile, efficacy and safety of lixisenatide in type 2 diabetes mellitus. Expert Opin. Pharmacother. 2013;14(16):2281–2296. doi: 10.1517/14656566.2013.838559. [DOI] [PubMed] [Google Scholar]
- 239.Christensen M., et al. The design and discovery of lixisenatide for the treatment of type 2 diabetes mellitus. Expert Opin. Drug Discov. 2014;9(10):1223–1251. doi: 10.1517/17460441.2014.942638. [DOI] [PubMed] [Google Scholar]
- 240.Horowitz M., Rayner C.K., Jones K.L. Mechanisms and Clinical Efficacy of Lixisenatide for the Management of Type 2 Diabetes. Adv. Ther. 2013;30(2):81–101. doi: 10.1007/s12325-013-0009-4. [DOI] [PubMed] [Google Scholar]
- 241.Roca-Rodríguez M.M., et al. Lixisenatida en pacientes con diabetes tipo 2 y obesidad: más allá del control glucémico. AtencióN. Prima. 2017;49(5):294–299. doi: 10.1016/j.aprim.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Whyte M.B., et al. Lixisenatide Reduces Chylomicron Triacylglycerol by Increased Clearance. J. Clin. Endocrinol. Metab. 2018;104(2):359–368. doi: 10.1210/jc.2018-01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kaneto H., et al. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination (iGlarLixi) in Japanese patients with type 2 diabetes mellitus inadequately controlled on basal insulin and oral antidiabetic drugs: The LixiLan JP-L randomized clinical trial. Diabetes, Obes. Metab. 2020;22(S4):3–13. doi: 10.1111/dom.14005. [DOI] [PubMed] [Google Scholar]
- 244.Riddle M.C., et al. Adding Once-Daily Lixisenatide for Type 2 Diabetes Inadequately Controlled by Established Basal Insulin: A 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36(9):2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rosenstock J., et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in Type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S) J. Diabetes its Complicat. 2014;28(3):386–392. doi: 10.1016/j.jdiacomp.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 246.Bolli G.B., et al. Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1) Diabet. Med. 2014;31(2):176–184. doi: 10.1111/dme.12328. [DOI] [PubMed] [Google Scholar]
- 247.Onishi Y., et al. Efficacy and safety of lixisenatide in Japanese patients with type 2 diabetes mellitus inadequately controlled by sulfonylurea with or without metformin: Subanalysis of GetGoal-S. J. Diabetes Investig. 2015;6(2):201–209. doi: 10.1111/jdi.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nauck M., et al. Once-Daily Liraglutide Versus Lixisenatide as Add-on to Metformin in Type 2 Diabetes: A 26-Week Randomized Controlled Clinical Trial. Diabetes Care. 2016;39(9):1501–1509. doi: 10.2337/dc15-2479. [DOI] [PubMed] [Google Scholar]
- 249.Ahrén B., et al. Efficacy and Safety of Lixisenatide Once-Daily Morning or Evening Injections in Type 2 Diabetes Inadequately Controlled on Metformin (GetGoal-M) Diabetes Care. 2013;36(9):2543–2550. doi: 10.2337/dc12-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rosenstock J., et al. Efficacy and Safety of Lixisenatide Once Daily Versus Exenatide Twice Daily in Type 2 Diabetes Inadequately Controlled on Metformin: A 24-week, randomized, open-label, active-controlled study (GetGoal-X) Diabetes Care. 2013;36(10):2945–2951. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hunt B., et al. Long-term Cost-effectiveness of Two GLP-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus in the Italian Setting: Liraglutide Versus Lixisenatide. Clin. Ther. 2017;39(7):1347–1359. doi: 10.1016/j.clinthera.2017.05.354. [DOI] [PubMed] [Google Scholar]
- 252.Leon N., et al. Lixisenatide (Adlyxin): A Once-Daily Incretin Mimetic Injection for Type-2 Diabetes. 2017;42(11):676–711. (P t) [PMC free article] [PubMed] [Google Scholar]
- 253.Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol. Cell. Endocrinol. 2009;297(1):137–140. doi: 10.1016/j.mce.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 254.Madsen K., et al. Structure−Activity and Protraction Relationship of Long-Acting Glucagon-like Peptide-1 Derivatives: Importance of Fatty Acid Length, Polarity, and Bulkiness. J. Med. Chem. 2007;50(24):6126–6132. doi: 10.1021/jm070861j. [DOI] [PubMed] [Google Scholar]
- 255.Wilding J.P.H., et al. Exposure–response analyses of liraglutide 3.0 mg for weight management. Diabetes, Obes. Metab. 2016;18(5):491–499. doi: 10.1111/dom.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Manigault K.R., Thurston M.M. Liraglutide: A Glucagon-Like Peptide-1 Agonist for Chronic Weight Management. Consult Pharm. 2016;31(12):685–697. doi: 10.4140/TCP.n.2016.685. [DOI] [PubMed] [Google Scholar]
- 257.Nuffer W.A., Trujillo J.M. Liraglutide: A New Option for the Treatment of Obesity. Pharmacother.: J. Hum. Pharmacol. Drug Ther. 2015;35(10):926–934. doi: 10.1002/phar.1639. [DOI] [PubMed] [Google Scholar]
- 258.Zhu E., et al. Liraglutide suppresses obesity and induces brown fat-like phenotype via Soluble Guanylyl Cyclase mediated pathway in vivo and in vitro. Oncotarget. 2016;7(49):81077–81089. doi: 10.18632/oncotarget.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pinelli N.R., Hurren K.M. Efficacy and Safety of Long-Acting Glucagon-Like Peptide-1 Receptor Agonists Compared with Exenatide Twice Daily and Sitagliptin in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Ann. Pharmacother. 2011;45(7-8):850–860. doi: 10.1345/aph.1Q024. [DOI] [PubMed] [Google Scholar]
- 260.Astrup A., et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2012;36(6):843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Astrup A., et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 262.Lean M.E.J., et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int. J. Obes. 2014;38(5):689–697. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.le Roux C.W., et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 264.Pi-Sunyer X., et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 265.Wadden T.A., et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int. J. Obes. 2013;37(11):1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 266.Bode B. Liraglutide: a review of the first once-daily GLP-1 receptor agonist. Am. J. Manag. care. 2011;17(. 2):S59–S70. [PubMed] [Google Scholar]
- 267.Knudsen L.B., Lau J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019;10 doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Christou G.A., et al. Semaglutide as a promising antiobesity drug. Obes. Rev. 2019;20(6):805–815. doi: 10.1111/obr.12839. [DOI] [PubMed] [Google Scholar]
- 269.Ingelfinger J.R., Rosen C.J. STEP 1 for Effective Weight Control — Another First Step? N. Engl. J. Med. 2021;384(11):1066–1067. doi: 10.1056/NEJMe2101705. [DOI] [PubMed] [Google Scholar]
- 270.Rosenstock J., et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA. 2019;321(15):1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Davies M., et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 272.Wilding J.P.H., et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 273.Rubino D.M., et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138–150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wadden T.A., et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cowart K. Oral Semaglutide: first-in-class Oral GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. Ann. Pharmacother. 2020;54(5):478–485. doi: 10.1177/1060028019889064. [DOI] [PubMed] [Google Scholar]
- 276.Gerstein H.C., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 277.Østergaard L., Frandsen C.S., Madsbad S. Treatment potential of the GLP-1 receptor agonists in type 2 diabetes mellitus: a review. Expert Rev. Clin. Pharmacol. 2016;9(2):241–265. doi: 10.1586/17512433.2016.1121808. [DOI] [PubMed] [Google Scholar]
- 278.Jendle J., et al. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes/Metab. Res. Rev. 2016;32(8):776–790. doi: 10.1002/dmrr.2810. [DOI] [PubMed] [Google Scholar]
- 279.Barrington P., et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes, Obes. Metab. 2011;13(5):426–433. doi: 10.1111/j.1463-1326.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 280.Dungan K.M., et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–1357. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 281.Umpierrez G., et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37(8):2168–2176. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 282.Frias J.P., et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11) Diabetes Care. 2021;44(3):765–773. doi: 10.2337/dc20-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Giorgino F., et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2) Diabetes Care. 2015;38(12):2241–2249. doi: 10.2337/dc14-1625. [DOI] [PubMed] [Google Scholar]
- 284.Zhou Y., et al. Pancreatic safety of once-weekly dulaglutide in Chinese patients with type 2 diabetes mellitus: subgroup analysis by potential influencing factors. Diabetes Ther. 2021;12(10):2677–2690. doi: 10.1007/s13300-021-01139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Meier J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 286.Drucker D.J., et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 287.Norwood P., et al. Safety of exenatide once weekly in patients with type 2 diabetes mellitus treated with a thiazolidinedione alone or in combination with metformin for 2 years. Clin. Ther. 2012;34(10):2082–2090. doi: 10.1016/j.clinthera.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 288.Elkind-Hirsch K.E., et al. Exenatide, dapagliflozin, or phentermine/topiramate differentially affect metabolic profiles in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2021;106(10):3019–3033. doi: 10.1210/clinem/dgab408. [DOI] [PubMed] [Google Scholar]
- 289.Malone J., et al. Exenatide once weekly for the treatment of type 2 diabetes. Expert Opin. Investig. Drugs. 2009;18(3):359–367. doi: 10.1517/13543780902766802. [DOI] [PubMed] [Google Scholar]
- 290.Zhang Y., et al. Protective effects and mechanisms of polyethylene glycol loxenatide against hyperglycemia and liver injury in db/db diabetic mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.781856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang G.-R., et al. Pharmacokinetics and pharmacodynamics of a polyethylene glycol (PEG)-conjugated GLP-receptor agonist once weekly in Chinese patients with type 2 diabetes. J. Clin. Pharmacol. 2015;55(2):152–158. doi: 10.1002/jcph.386. [DOI] [PubMed] [Google Scholar]
- 292.Guo Z., et al. PEX-168 improves insulin resistance, inflammatory response and adipokines in simple obese mice: a mechanistic exploration. BMC Endocr. Disord. 2021;21(1):245. doi: 10.1186/s12902-021-00908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Seino Y., Fukushima M., Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J. Diabetes Investig. 2010;1(1-2):8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kleinert M., et al. Glucagon regulation of energy expenditure. Int. J. Mol. Sci. 2019;20(21):5407. doi: 10.3390/ijms20215407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhao F., et al. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nat. Commun. 2022;13(1):1057. doi: 10.1038/s41467-022-28683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Knerr P.J., et al. Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice. Mol. Metab. 2022;63 doi: 10.1016/j.molmet.2022.101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gault V.A., et al. A novel glucagon-like peptide-1 (GLP-1)/glucagon hybrid peptide with triple-acting agonist activity at glucose-dependent insulinotropic polypeptide, GLP-1, and glucagon receptors and therapeutic potential in high fat-fed mice. J. Biol. Chem. 2013;288(49):35581–35591. doi: 10.1074/jbc.M113.512046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nauck M.A. and D.A. D‘Alessio, Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022;21(1):169. doi: 10.1186/s12933-022-01604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Karagiannis T., et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65(8):1251–1261. doi: 10.1007/s00125-022-05715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is presented in the article.