Abstract
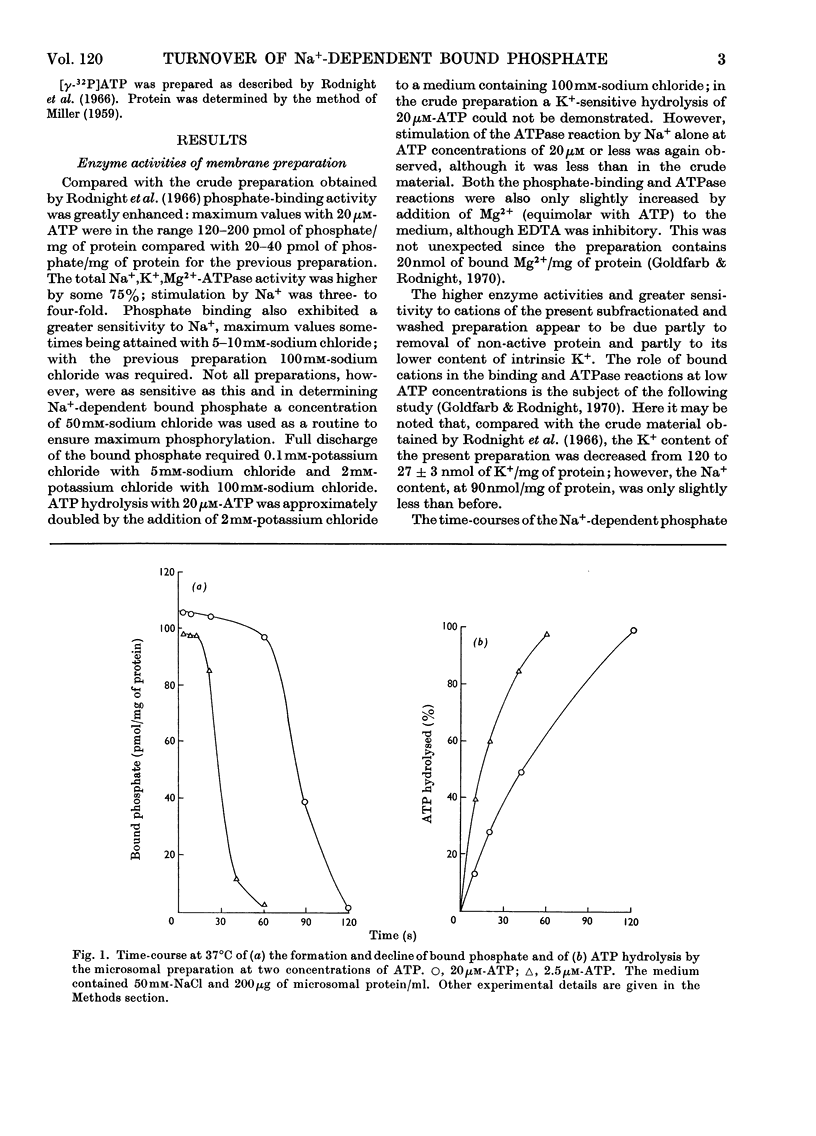
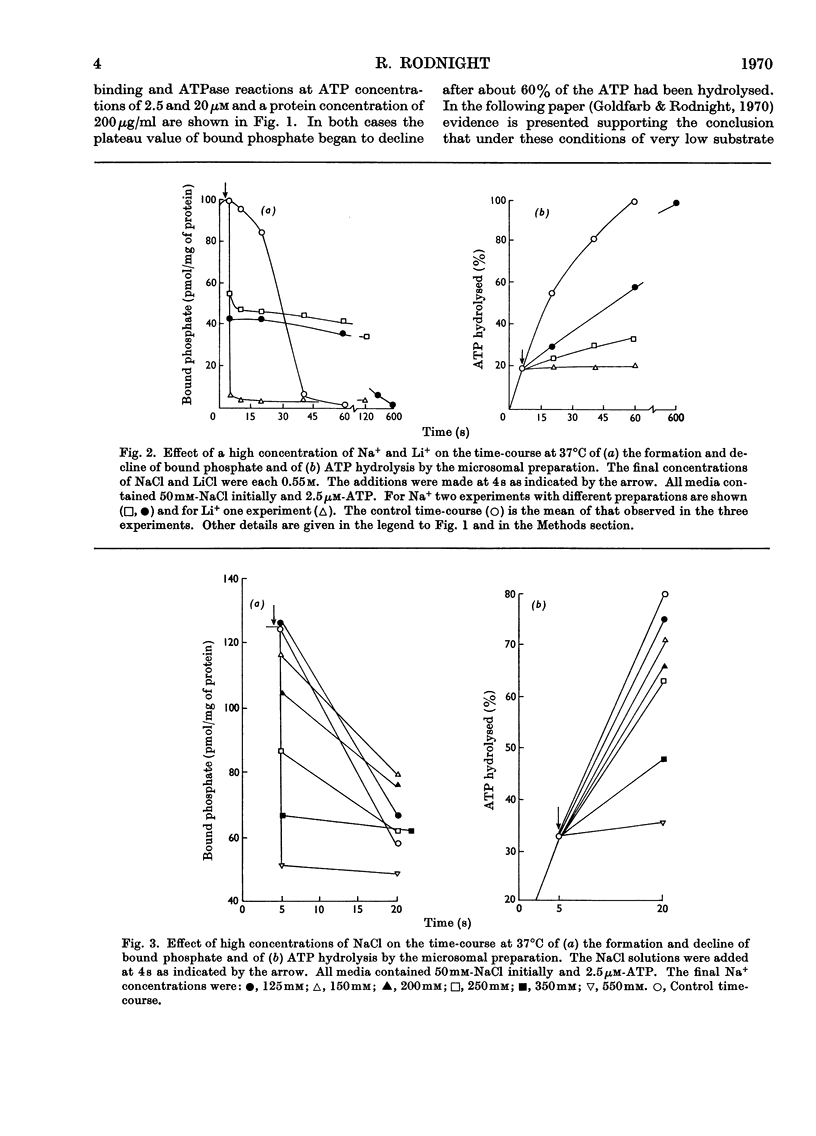
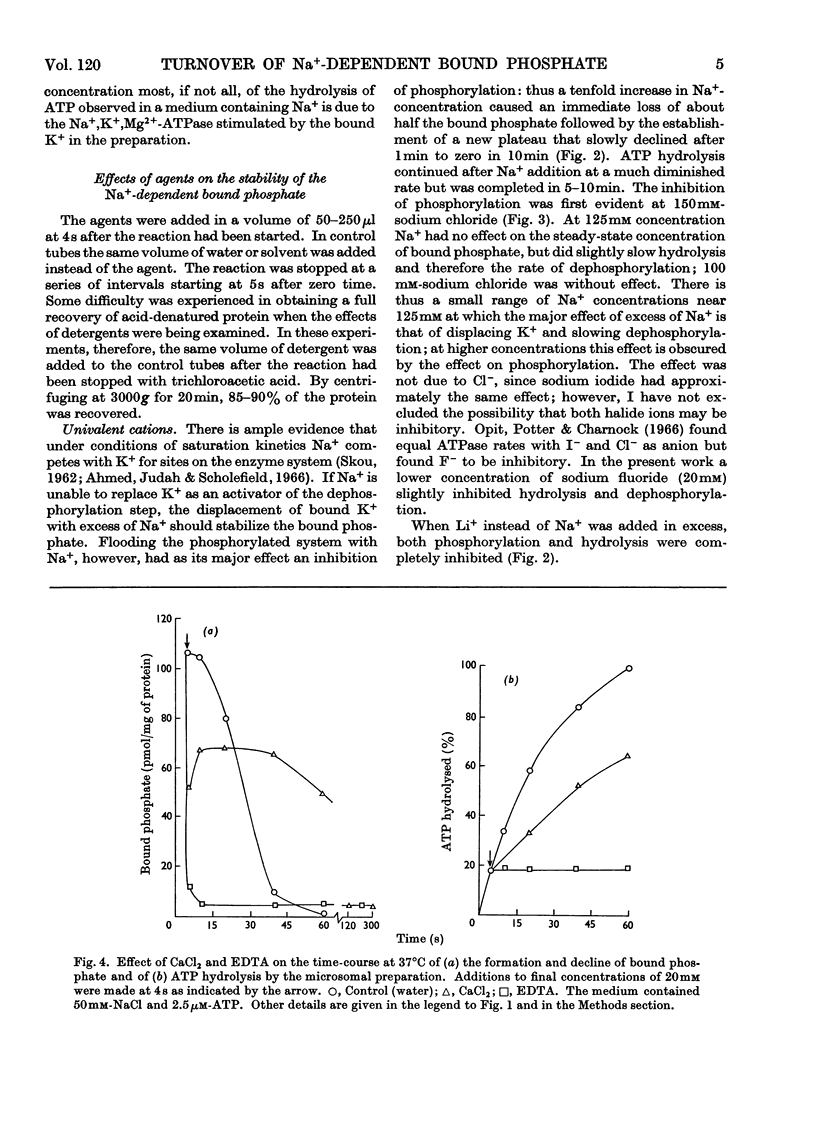
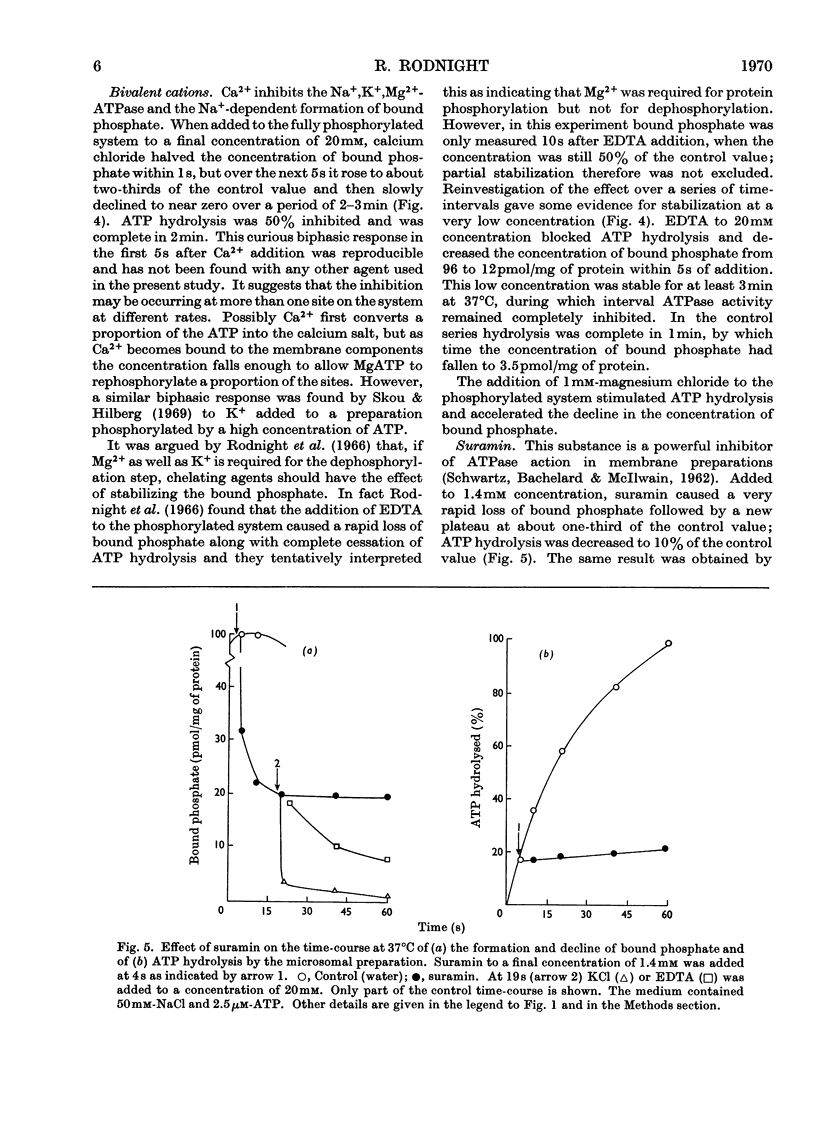
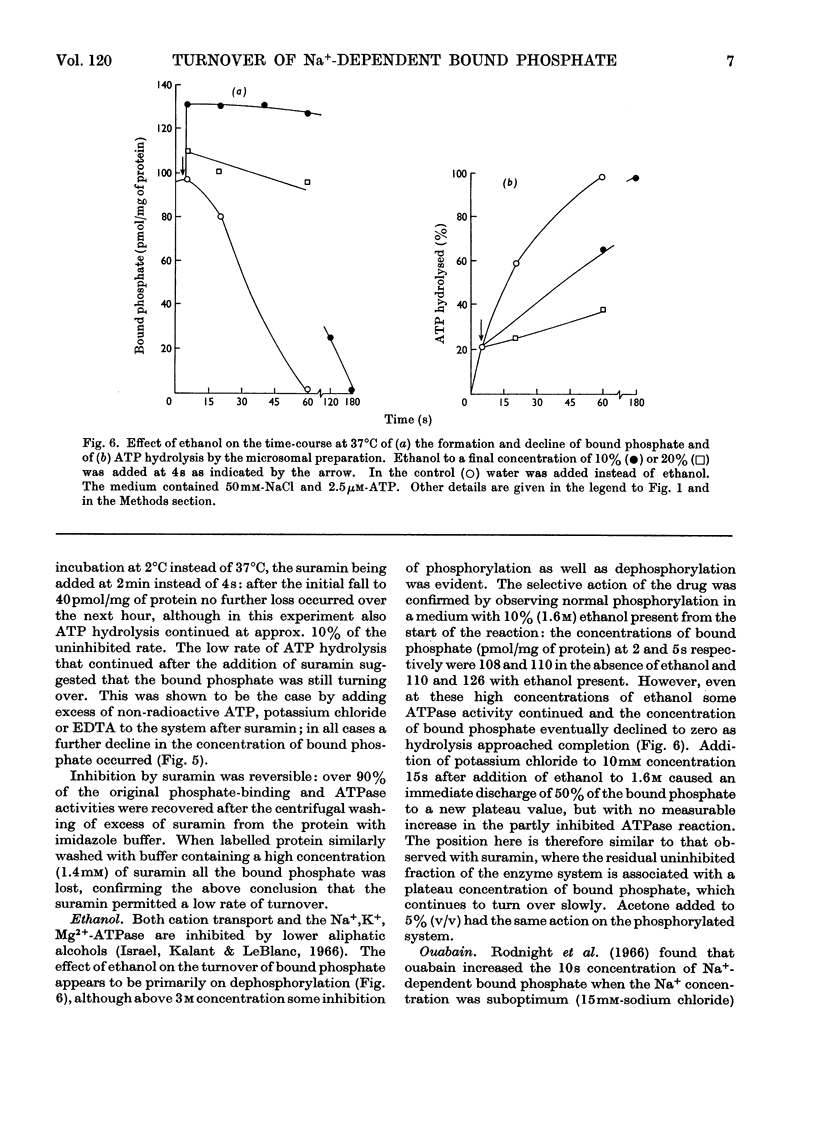
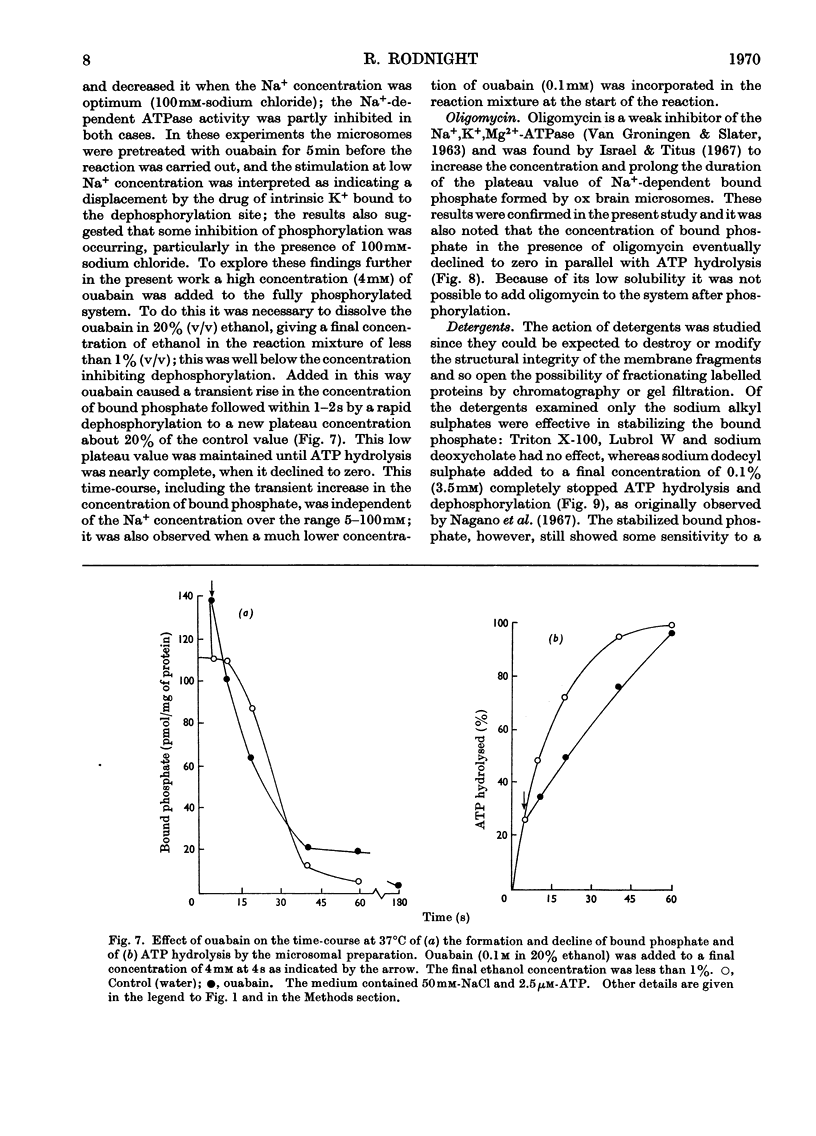
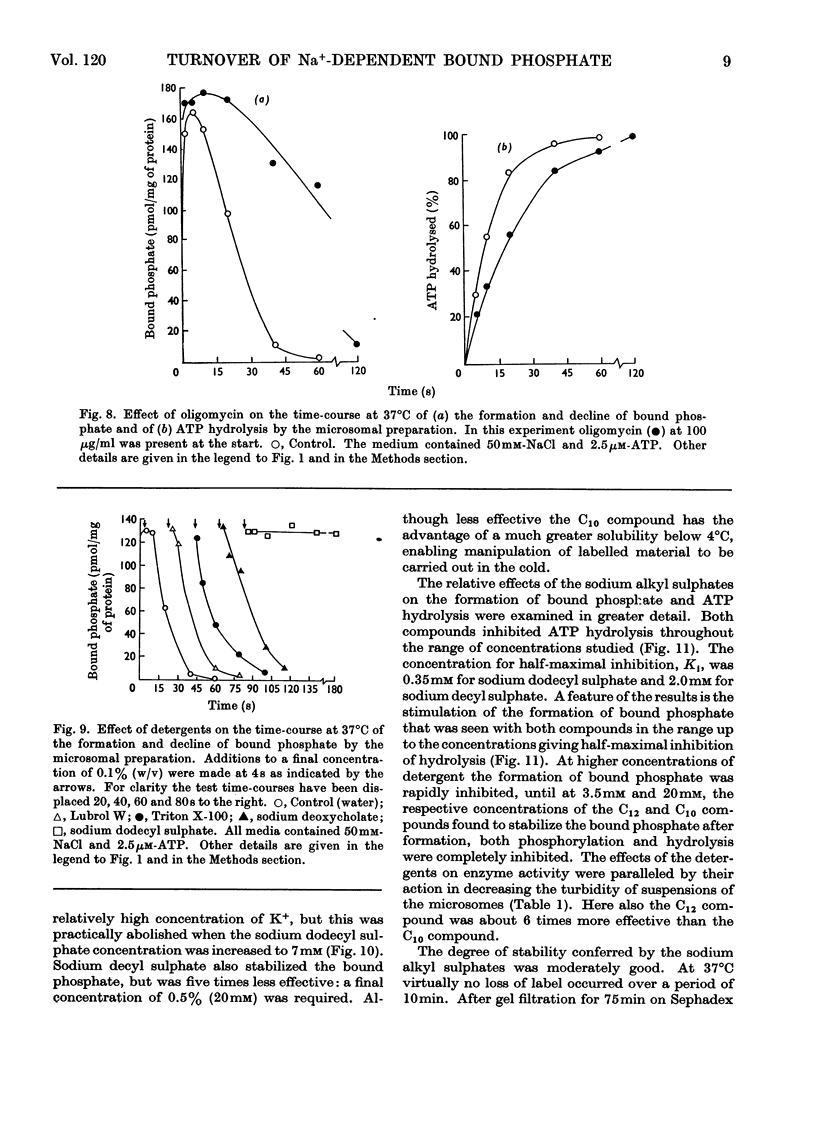
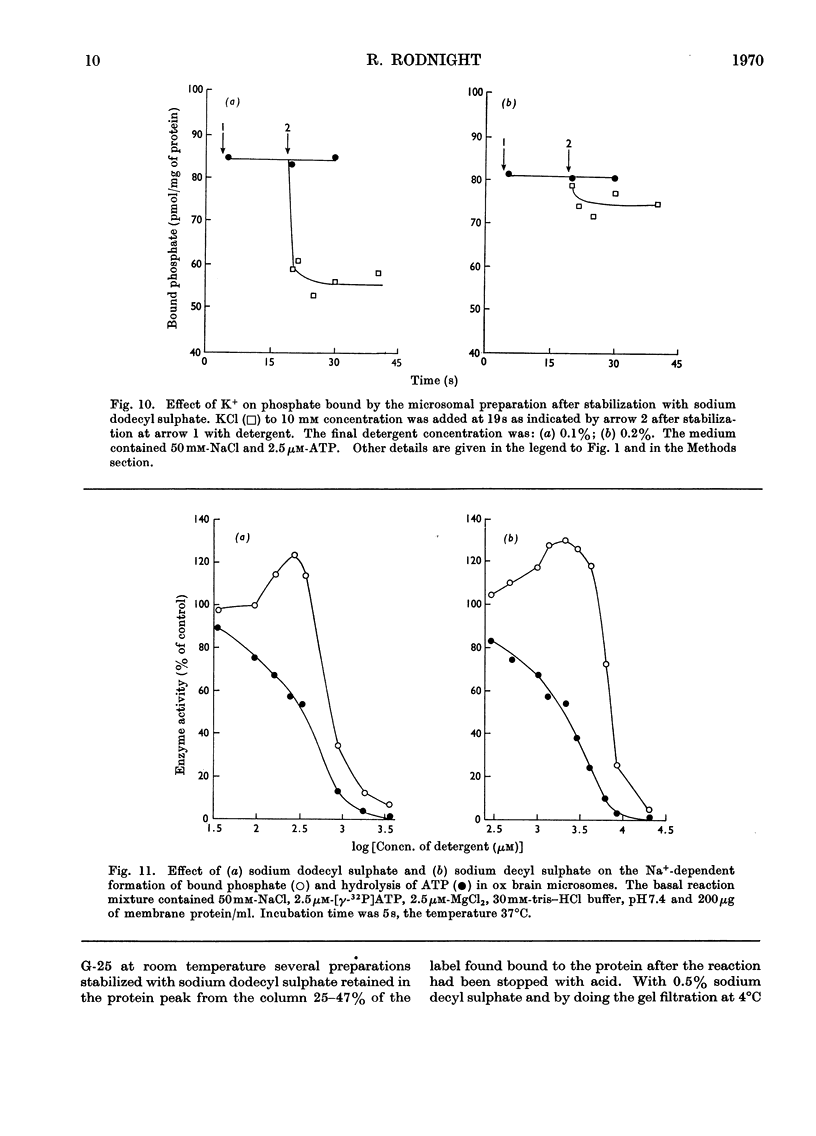
1. The effect of chemical agents on the turnover of the Na+-dependent bound phosphate and the simultaneous Na+-dependent hydrolysis of ATP by a membrane preparation from ox brain was studied at an ATP/protein ratio of 12.5pmol/μg. 2. The agents were added immediately after phosphorylation of the preparation in a medium containing 50mm-sodium chloride and 2.5μm-[γ-32P]ATP. 3. Concentrations of sodium chloride above 150mm, calcium chloride to 20mm and suramin to 1.4mm inhibited both phosphorylation and dephosphorylation and concomitantly slowed ATP hydrolysis. At 125mm-sodium chloride dephosphorylation and hydrolysis were slightly slowed without affecting phosphorylation. 4. Ethanol to 1.6m concentration inhibited dephosphorylation without affecting phosphorylation; the bound phosphate was increased and ATP hydrolysis slowed. 5. Ouabain to 4mm concentration partially inhibited ATP hydrolysis and caused a transient (1–2s) rise in bound phosphate followed by a rapid fall to a lower plateau value, which eventually declined to zero by the time ATP hydrolysis was complete. 6. Of the detergents examined Lubrol W, Triton X-100 and sodium deoxycholate had no significant effect on turnover. Sodium dodecyl sulphate and sodium decyl sulphate to 3.5mm and 20mm respectively completely inhibited turnover and ATP hydrolysis and stabilized the bound phosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Judah J. D., Scholefield P. G. Interaction of sodium and potassium with a cation-dependent adenosine triphosphatase system from rat brain. Biochim Biophys Acta. 1966 Jul 13;120(3):351–360. doi: 10.1016/0926-6585(66)90302-5. [DOI] [PubMed] [Google Scholar]
- Albers R. W., Koval G. J., Siegel Studies on the interaction of ouabain and other cardio-active steroids with sodium-potassium-activated adenosine triphosphatase. Mol Pharmacol. 1968 Jul;4(4):324–336. [PubMed] [Google Scholar]
- Fahn S., Hurley M. R., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. II. Effects of N-ethylmaleimide and other sulfhydryl reagents. J Biol Chem. 1966 Apr 25;241(8):1890–1895. [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem. 1966 Apr 25;241(8):1882–1889. [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. V. Phosphorylation by adenosine triphosphate-32P. J Biol Chem. 1968 Apr 25;243(8):1993–2002. [PubMed] [Google Scholar]
- Goldfarb P. S., Rodnight R. The role of bound potassium ions in the hydrolysis of low concentrations of adenosine triphosphate by preparations of membrane fragments from ox brain cerebral cortex. Biochem J. 1970 Nov;120(1):15–24. doi: 10.1042/bj1200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y., Titus E. A comparison of microsomal (Na+ + K+)-ATPase with K+-acetylphosphatase. Biochim Biophys Acta. 1967 Jul 11;139(2):450–459. doi: 10.1016/0005-2744(67)90048-4. [DOI] [PubMed] [Google Scholar]
- Isreal Y., Kalant H., LeBlanc A. E. Effects of lower alcohols on potassium transport and microsomal adenosine-triphosphatase activity of rat cerebral cortex. Biochem J. 1966 Jul;100(1):27–33. doi: 10.1042/bj1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg A., Galsworthy P. R., Hokin L. E. Sodium-potassium adenosine triphosphatase: acyl phosphate "intermediate" shown to be L-glutamyl-gamma-phosphate. Science. 1967 Jul 28;157(3787):434–436. doi: 10.1126/science.157.3787.434. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Saito M., Tonomura Y. [Properties of a phosphorylated protein as a reaction intermediate of Na+-K+ sensitive ATPase]. J Biochem. 1967 May;61(5):555–566. doi: 10.1093/oxfordjournals.jbchem.a128586. [DOI] [PubMed] [Google Scholar]
- Mnagano K., Mizuno N., Fujita M., Tashima Y., Nakao T., Nakao M. On the possible role of the phosphorylated intermediate in the reaction mechanism of (Na+-K+)-ATPase. Biochim Biophys Acta. 1967 Jul 5;143(1):239–248. doi: 10.1016/0005-2728(67)90124-7. [DOI] [PubMed] [Google Scholar]
- Opit L. J., Potter H., Charnock J. S. The effect of anions on (Na+ + K+)-activated ATPase. Biochim Biophys Acta. 1966 May 12;120(1):159–161. doi: 10.1016/0926-6585(66)90288-3. [DOI] [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Rodnight R., Hems D. A., Lavin B. E. Phosphate binding by cerebral microsomes in relation to adenosine-triphosphatase activity. Biochem J. 1966 Nov;101(2):502–515. doi: 10.1042/bj1010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnight R., Lavin B. E. Enzyme transfer of phosphate from adenosine triphosphate to protein-bound serine residues in cerebral microsomes. Biochem J. 1966 Nov;101(2):495–501. doi: 10.1042/bj1010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. Preparation from mammallian brain and kidney of the enzyme system involved in active transport of Na ions and K ions. Biochim Biophys Acta. 1962 Apr 9;58:314–325. doi: 10.1016/0006-3002(62)91015-6. [DOI] [PubMed] [Google Scholar]
- Schoner W., Beusch R., Kramer R. On the mechanism of Na plus and K plus-stimulated hydrolysis of adenosine triphosphate. 2. Comparison of nucleotide specificities of Na plus and K plus-activated ATPase and Na plus-dependent phosphorylation of cell membranes. Eur J Biochem. 1968 Dec;7(1):102–110. doi: 10.1111/j.1432-1033.1968.tb19580.x. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Hilberg C. The effect of cations, g-strophanthin and oligomycin on the labeling from [32P] ATP of the (Na+ + K+)-activated enzyme system and the effect of cations and g-strophanthin on the labeling from [32P] ITP and 32Pi. Biochim Biophys Acta. 1969 Jul 8;185(1):198–219. doi: 10.1016/0005-2744(69)90295-2. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Hilberg C. The effect of sulphydryl-blocking reagents and of urea on the (Na+ + K+)-activated enzyme system. Biochim Biophys Acta. 1965 Nov 22;110(2):359–369. doi: 10.1016/s0926-6593(65)80043-1. [DOI] [PubMed] [Google Scholar]
- VAN GRONINGENH, SLATER E. C. THE EFFECT OF OLIGOMYCIN ON THE (NA+ + K+)-ACTIVATED MAGNESIUM ATPASE OF BRAIN MICROSOMES AND ERYTHROCYTE MEMBRANE. Biochim Biophys Acta. 1963 Jul 9;73:527–530. doi: 10.1016/0006-3002(63)90460-8. [DOI] [PubMed] [Google Scholar]


