Abstract
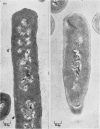
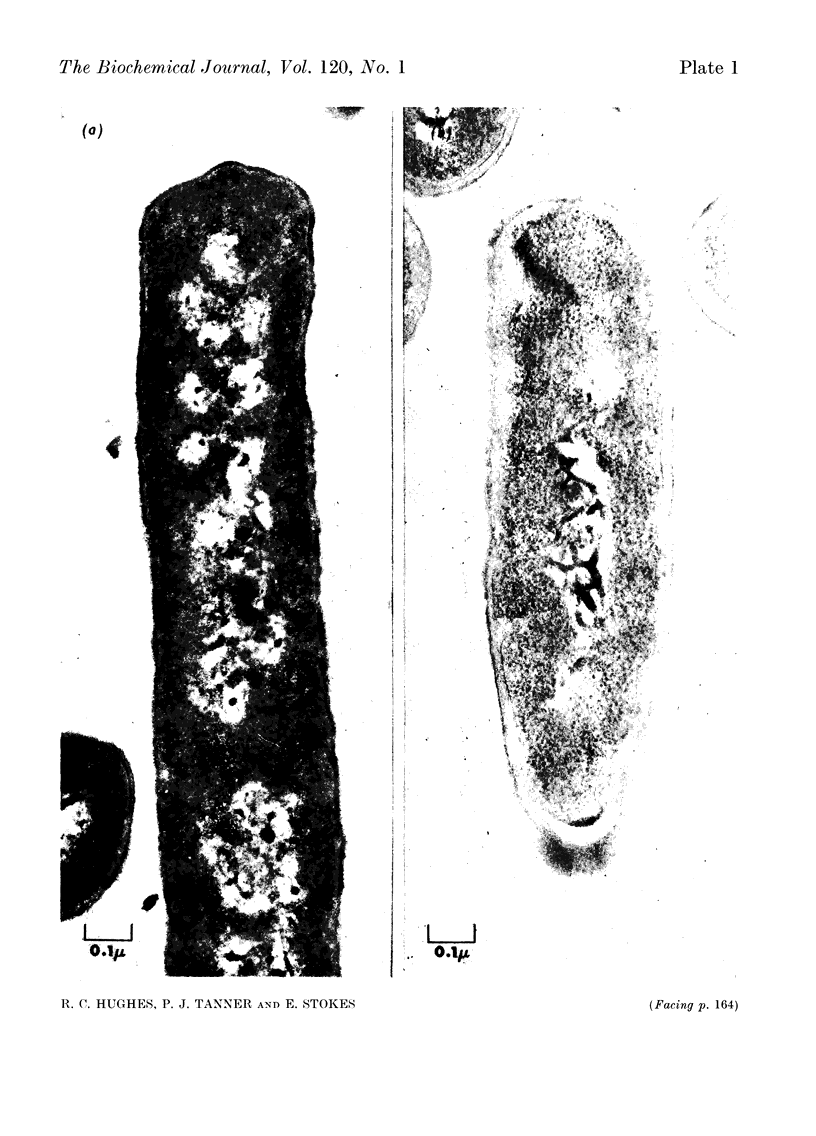
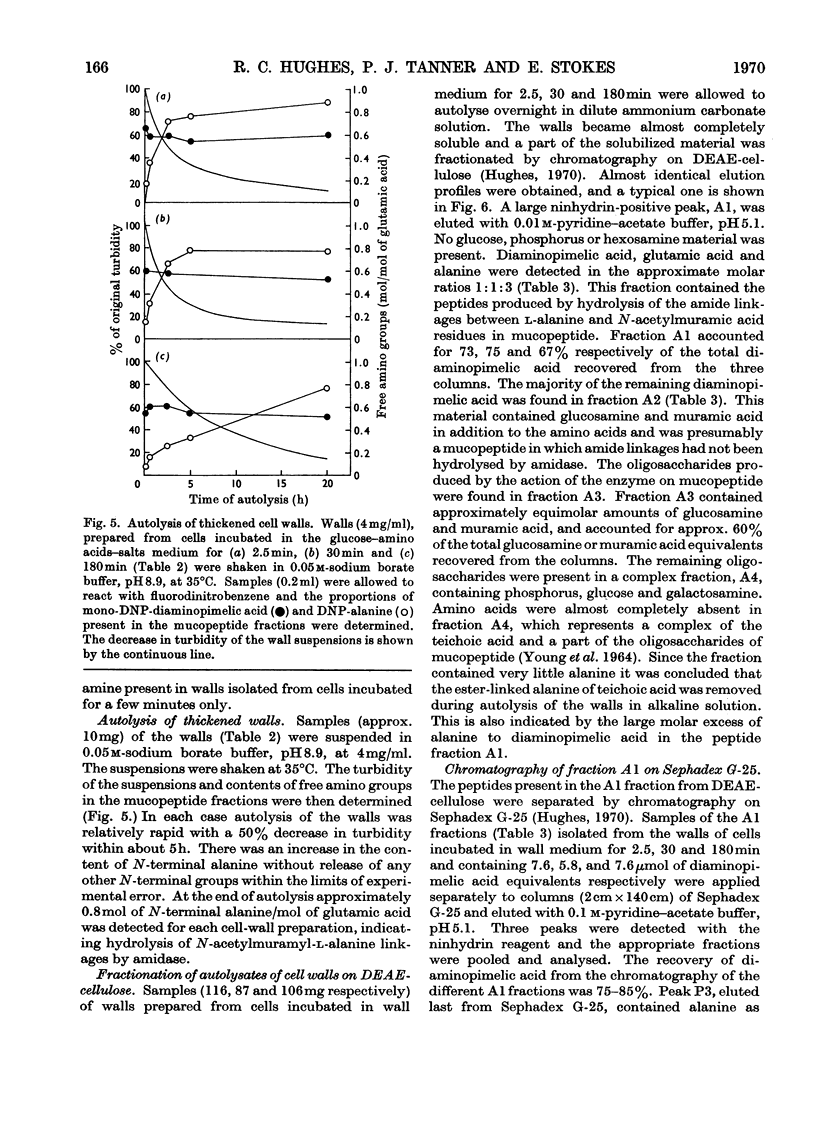
1. Incubation of Bacillus subtilis 168 trp in a glucose–amino acids–salts medium lacking tryptophan leads to an inhibition of cellular growth without affecting cell-wall synthesis. The cell walls increased approximately two- to three-fold in thickness and at the same time the amount of mucopeptide in the cells measured chemically increased to about the same extent. 2. Synthesis of mucopeptide and teichoic acid as measured by the extent of incorporation of radioactivity continued linearly for approximately 1h and then stopped. No reason was found for the strictly limited synthesis of the wall polymers. 3. The initial rates of incorporation of [32P]Pi or [3H]alanine into teichoic acid and of 3H-labelled amino acids into mucopeptide were not appreciably inhibited by the addition of chloramphenicol to the glucose–amino acids–salts medium. 4. There was no selective turnover of the mucopeptide synthesized by the cells in a medium lacking tryptophan on resumption of growth in a complete medium. 5. Wall synthesis taking place during the thickening process was similar to normal wall synthesis proceeding in growing cells. Walls of different thicknesses prepared from cells incubated for various times in incomplete medium did not differ qualitatively in composition. The products of autolysis of thickened walls were isolated and the analyses indicated a close similarity in the details of their mucopeptide structure compared with the mucopeptide of cells growing in the exponential phase.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku N., Landman O. E. Control of the synthesis of macromolecules during amino acid and thymine starvation in Bacillus subtilis. J Bacteriol. 1968 May;95(5):1813–1827. doi: 10.1128/jb.95.5.1813-1827.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Young F. E. Dynamic interactions between cell wall polymers, extracellular proteases and autolytic enzymes. Biochem Biophys Res Commun. 1970 Feb 20;38(4):564–568. doi: 10.1016/0006-291x(70)90618-2. [DOI] [PubMed] [Google Scholar]
- CHUNG K. L., HAWIRKO R. Z., ISAAC P. K. CELL WALL REPLICATION. I. CELL WALL GROWTH OF BACILLUS CEREUS AND BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:43–48. doi: 10.1139/m64-007. [DOI] [PubMed] [Google Scholar]
- Chung K. L. Autoradiographic studies of bacterial cell wall replication. I. Cell wall growth of Bacillus cereus in the presence of chloramphenicol. Can J Microbiol. 1967 Apr;13(4):341–350. doi: 10.1139/m67-046. [DOI] [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. C. Regulation of chromosome replication in Bacillus subtilis: effects of amino acid starvation in strain 168. J Bacteriol. 1969 Sep;99(3):730–736. doi: 10.1128/jb.99.3.730-736.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINDLAY J., LEVVY G. A. Purification of beta-N-acetylglucosaminidase from the pig epididymis. Biochem J. 1960 Oct;77:170–175. doi: 10.1042/bj0770170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK R., PARK J. T. Cell-wall synthesis by Staphylococcus aureus in the presence of chloramphenicol. Nature. 1958 Apr 12;181(4615):1050–1052. doi: 10.1038/1811050a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. Autolysis of isolated cell walls of Bacillus licheniformis N.C.T.C. 6346 and Bacillus subtilis Marburg Strain 168. Separation of the products and characterization of the mucopeptide fragments. Biochem J. 1970 Oct;119(5):849–860. doi: 10.1042/bj1190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Pavlik J. G., Rogers H. J., Tanner P. J. Organization of polymers in the cell walls of some bacilli. Nature. 1968 Aug 10;219(5154):642–644. doi: 10.1038/219642a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Composition of the mucopeptide component. Biochem J. 1968 Jan;106(1):41–48. doi: 10.1042/bj1060041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. Chloramphenicol-resistant incorporation of amino-acids into Staphylococci and cell-wall synthesis. Nature. 1958 Apr 5;181(4614):956–957. doi: 10.1038/181956a0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. The incorporation of amino acids into the cell-wall mucopeptide of staphylococci and the effect of antibiotics on the process. Biochem J. 1959 Aug;72:654–662. doi: 10.1042/bj0720654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Rogers H. J. The inhibition of mucopeptide synthesis by benzylpenicillin in relation to irreversible fixation of the antibiotic by staphylococci. Biochem J. 1967 Apr;103(1):90–102. doi: 10.1042/bj1030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORK E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of alpha epsilon-diaminopimelic acid. Biochem J. 1957 Nov;67(3):416–423. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt P. J. Cell wall thickness, size distribution, refractive index ratio and dry weight content of living bacteria (Staphylococcus aureus). Nature. 1970 Apr 18;226(5242):277–279. doi: 10.1038/226277a0. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J., CRAWFORD I. P. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. I. CHEMICAL COMPOSITION OF CELL WALLS. J Biol Chem. 1963 Sep;238:3119–3125. [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]
- Young F. E. Variation in the chemical composition of the cell walls of Bacillus subtilis during growth in different media. Nature. 1965 Jul 3;207(992):104–105. doi: 10.1038/207104b0. [DOI] [PubMed] [Google Scholar]