Abstract
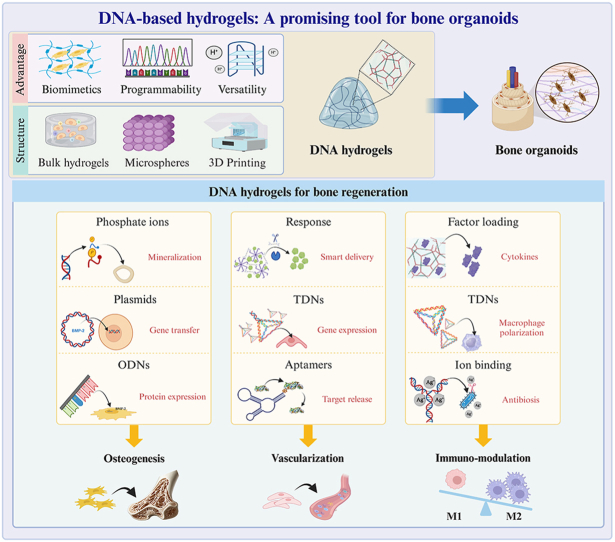
DNA-based hydrogels stand out for bone regeneration due to their exceptional biocompatibility and programmability. These hydrogels facilitate the formation of spatial bone structures through bulk hydrogel fabricating, microsphere formatting, and 3D printing. Furthermore, the bone microenvironment can be finely tuned by leveraging the degradation products, nanostructure, targeting, and delivery capabilities inherent to DNA-based materials. In this review, we underscore the advantages of DNA-based hydrogels, detailing their composition, gelation techniques, and structure optimization. We then delineate three critical elements in the promotion of bone regeneration using DNA-based hydrogels: (i) osteogenesis driven by phosphate ions, plasmids, and oligodeoxynucleotides (ODNs) that enhance mineralization and promote gene and protein expression; (ii) vascularization facilitated by tetrahedral DNA nanostructures (TDNs) and aptamers, which boosts gene expression and targeted release; (iii) immunomodulation achieved through loaded factors, TDNs, and bound ions that stimulate macrophage polarization and exhibit antibacterial properties. With these advantages and properties, these DNA-based hydrogels can be used to construct bone organoids, providing an innovative tool for disease modeling and therapeutic applications in bone tissue engineering. Finally, we discuss the current challenges and future prospects, emphasizing the potential impacts and applications in regenerative medicine.
Keywords: DNA-Based hydrogels, Bone organoids, Osteogenesis, Vascularization, Immno-modulation
Graphical abstract
1. Introduction
Bone-related diseases, such as bone defects, osteoporosis, and osteoarthritis, present significant challenges to both health and quality of life [1,2]. These conditions can lead to reduced mobility, chronic pain, and an increased risk of fractures, significantly impacting daily functioning and overall well-being. In recent years, DNA-based hydrogels have emerged as a promising biomaterial for promoting bone regeneration. Their unique properties make them highly effective in supporting bone healing [3]. DNA-based hydrogels present significant advantages as biomaterials, particularly in medical applications [4]. Their high biomimicry is achieved because DNA-based is a naturally occurring molecule in the body, which minimizes the risk of immune reactions [5]. DNA can break down into nucleotides that can be safely metabolized. Their programmability allows for precise control of the hydrogel's structures and functions, enabling responses to environmental stimuli such as pH and DNAzyme [[6], [7], [8]]. Additionally, DNA-based hydrogels can be versatile, allowing for targeted effect toward cells and controlled drug release [9]. With advances in molecular biology, large-scale DNA synthesis has become feasible, supporting the scalability for clinical use [10]. DNA-based hydrogels can be used to create advanced spatial structures through various techniques such as microsphere formatting and 3D printing [11]. Their structural properties mimic the extracellular matrix to support cell adhesion, proliferation, and differentiation. These properties make DNA-based hydrogels an excellent choice for applications in regenerative medicine.
Bone regeneration can be stimulated through three primary pathways including osteogenesis, angiogenesis, and immune modulation by harnessing the distinctive properties of DNA-based hydrogels. DNA-based hydrogels enhance osteogenesis through the following approaches. As DNA-based hydrogels degrade, phosphate ions are released to promote the mineralization process [12]. Oligodeoxynucleotides (ODNs) and tetrahedral framework nucleic acids (tFNAs) enhance osteogenic differentiation, while plasmid DNA delivery stimulates bone formation [13]. Then, aptamers target bone marrow stem cells (BMSCs), and DNAzymes help clear reactive oxygen species (ROS) from the microenvironment, further supporting bone regeneration [14]. DNA-based hydrogels can also facilitate bone regeneration via angiogenesis. DNA-based hydrogels can incorporate the CRISPRa system to activate the expression angiogenesis factor, and tFNA can be introduced to further enhance vascularization [15]. Responsive aptamers can be added to enable controlled degradation of the hydrogels, ensuring a sustained release of agents. DNA-based hydrogels help coordinate the immune system's role in healing by optimizing both macrophage and T cell responses. DNA-based hydrogels can incorporate tFNA to modulate immune responses by promoting macrophage polarization. Functionalized DNA-based hydrogels have shown potential in regulating T cells, particularly by promoting the recruitment and differentiation of Treg cells [16]. DNA-based hydrogels can also degrade in response to the surrounding environment and modulate the immune microenvironment [17].
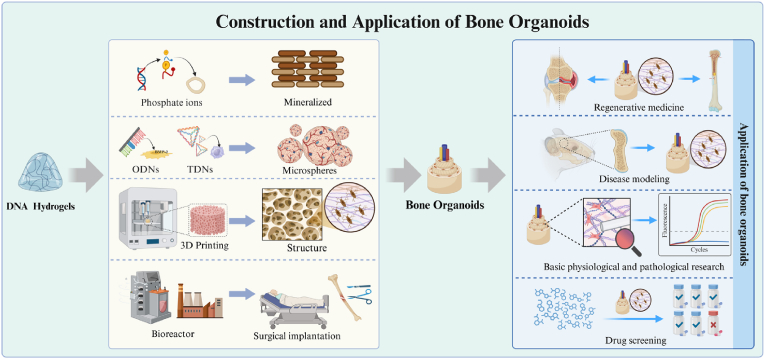
Bone organoids are three-dimensional, self-renewing, and self-organizing microstructures of bone tissue that emulate natural spatial characteristics [18]. These organoids are constructed from bioactive materials and are directionally differentiated from various stem cells or progenitor cells [19,20]. DNA-based hydrogels are emerging as an outstanding material for bone regeneration. Their biomimetic properties, programmability, and versatility enable them to effectively promote osteogenesis, vascularization, and immune modulation [21]. Additionally, DNA-based hydrogels can be used as bioinks in 3D bioprinting for constructing complex bone organoids that mimic the architecture of bone tissue [22]. The organoids hold immense potential in disease modeling, drug screening, and bone-related disease studies [23,24]. Thus, DNA-based hydrogels are a promising tool for bone organoids.
In this review, we highlight the benefits of DNA-based hydrogels, focusing on their composition, gelation methods, and structural optimization. We have further elaborated on the specific applications of DNA-based hydrogels in promoting bone regeneration through three key mechanisms: osteogenesis, vascularization, and immune modulation. With their advantages of biomimetics, programmability, and versatility, DNA-based hydrogels present a promising platform for constructing bone organoids, offering innovative solutions for disease modeling and therapeutic applications in bone tissue engineering. Furthermore, we discuss the current challenges of this technology and explore future opportunities, with highlighting its potential to revolutionize regenerative medicine and its broad applications in clinical settings (Fig. 1).
Fig. 1.
Schematic illustration of construction of bone organoids with DNA-based hydrogels.
2. Advantages of DNA-based hydrogels
DNA-based hydrogels offer unique advantages due to their biomimetics, programmability, and versatility [25,26]. As naturally occurring molecules, DNA-based hydrogels are highly biomimetics, reducing the risk of immune reactions and ensuring safe biodegradation in the body [27]. Functionally, DNA-based hydrogels can be customized with bioactive molecules for targeted therapies, allowing for controlled release of drugs or growth factors in response to environmental stimuli [28]. Their programmability, derived from DNA's sequence-specific hybridization, enables precise control over the assembly, disassembly, and responsiveness of the hydrogels, making them adaptable for a wide range of biomedical applications, such as tissue engineering and drug delivery [29]. The programmability of DNA-based hydrogels is one of the most powerful features, stemming from the inherent properties of DNA molecules. DNA can be precisely programmed through sequence-specific hybridization and gene editing, allowing the design of hydrogels with tailored structures and functions [30,31].
2.1. Biomimetics
DNA-based hydrogels have garnered significant attention in the biomedical field due to their unique biomimetics. This biomimetics is primarily attributed to several key factors [32]. The intrinsic biomimetics of DNA is largely attributed to its natural biological origin [33]. DNA, as the carrier of genetic information in all living organisms, is a naturally occurring biomolecule [34]. The natural origin of DNA-based hydrogels ensures that biomimicry is inherently achieved, as synthetic materials are not introduced into the body [35]. The body's immune system recognizes DNA as a familiar substance, reducing the likelihood of adverse reactions such as inflammation or immune rejection [36]. This natural compatibility allows DNA-based hydrogels to integrate seamlessly into biological environments, making them suitable for various applications [37]. Moreover, DNA-based hydrogels demonstrate a high degree of biodegradability. DNA-based hydrogels can be enzymatically degraded by nucleases, breaking down into nucleotides [38,39]. These degradation products can be further utilized by the body.
DNA-based hydrogels exhibit both structural and versatility biomimicry, making them a versatile material for biomedical applications [7,40]. DNA-based hydrogels can self-assemble into complex three-dimensional networks that mimic the microarchitecture of biological tissues [41,42]. This structural similarity allows them to provide mechanical support and facilitate cellular behaviors, such as cell attachment and proliferation, like the extracellular matrix in natural tissues [43,44]. These hydrogels can also be engineered to respond to specific environmental cues, mimicking the dynamic responses of biological materials to changes in their surroundings [45]. By combining unique biomimetic properties, DNA-based hydrogels offer a promising platform for developing advanced biomaterials that are both effective and safe for use in regenerative medicine and other biomedical applications [46,47].
2.2. Programmability
The programmability of DNA-based hydrogels is one of the most powerful features, stemming from the inherent properties of DNA molecules. DNA can be precisely programmed through sequence-specific hybridization and gene editing, allowing the design of hydrogels with tailored structures and functions [48]. This programmability enables the incorporation of various bioactive molecules, such as growth factors or therapeutic agents, into the hydrogel matrix and their release in a controlled manner [29,49]. Programming DNA sequences allows for precise control over the higher-order structures and functions of DNA nanomaterials, influencing the properties of hydrogels. Many advanced structural DNA nanomaterials have been created, including motif, TDNs [[50], [51], [52], [53]].
CRISPR-Cas9 is a crucial gene editing tool that exemplifies the programmability of DNA. This technology can enable precise genetic modifications [54,55]. By programming the guide RNA (gRNA) to target a specific DNA sequence, CRISPR-Cas9 can accurately direct the Cas9 enzyme to cut and edit the genome at the desired location [56]. Theoretically, this system can remodel almost any DNA sequence in the genome by changing the sgRNA sequence, making CRISPR-Cas9 one of the most powerful gene editing tools. Its applications include generating gene knock-out cell lines or animal models [56,57]. Zhang et al. developed a non-ionic nanogel-based CRISPR/Cas9 delivery platform, with Cas9/sgRNA-loaded DNA-g-PCL brushes crosslinked via DNA ligation, forming nanogels through nucmwisleic acid hybridization [58]. This enabled effective intracellular delivery of Cas9/sgRNA complexes for targeted gene editing.
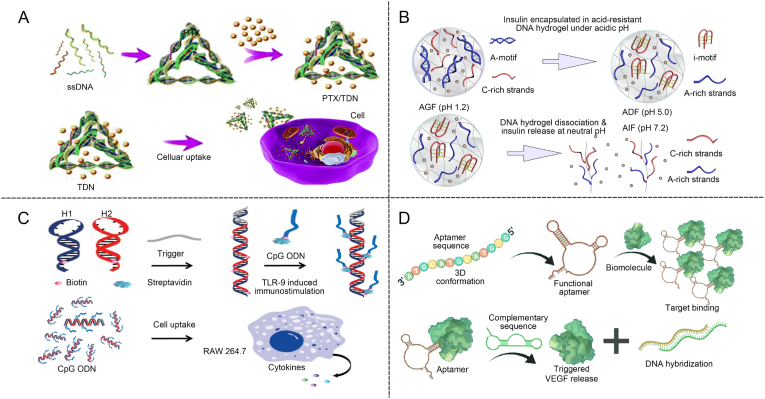
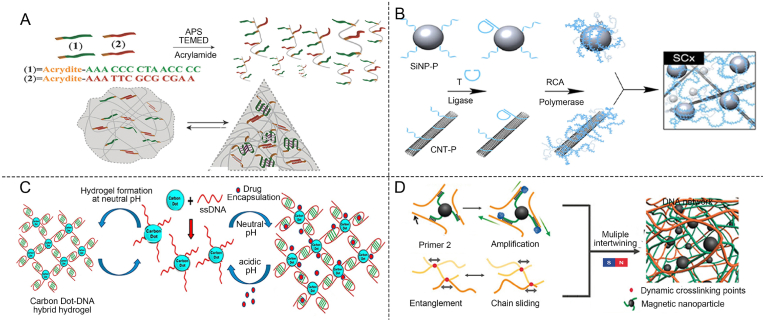
TDNs are formed from four highly specific and programmable ssDNA molecules [59,60]. They offer outstanding biomimetics, programmability, and abundant sites for functional modifications. Additionally, they possess robust mechanical rigidity and high stability [61]. DNA tetrahedra-based delivery carriers with active targeting effects, facilitate stable drug encapsulation and stimulus-responsive release on demand, providing a universal platform for various drug delivery needs [[62], [63], [64]]. Lin et al. modified TDNs with oligonucleotide ends through chemical modification (Fig. 2A) [65]. TDN entered and exited cells via endocytosis and exocytosis pathways that bypass drug efflux pumps, making it an effective carrier for delivering therapeutic agent paclitaxel (PTX) to multidrug-resistant cells.
Fig. 2.
Advantages and applications of DNA. (A) Schematic diagram of TDNs for intracellular delivery of encapsulated drugs [65]. Copyright 2018, Reproduced with permission. (B) Schematic of pH-regulated DNA-based hydrogel changes to form a motif structure for controlled drug release [74]. Copyright 2022, Reproduced with permission. (C) Schematic of immunity regulation using CpG ODN through macrophage secretion of cytokines [81]. Copyright 2017, Reproduced with permission. (D) Spatial and temporal regulation of chemical antibodies for promoting vascular regeneration [88]. Copyright 2023, Reproduced with permission.
Additionally, DNA can be programmed to form specific structural motifs to construct DNA-based hydrogels by designing sequences that undergo predictable hybridization through base pairing [66]. Watson-Crick base pairing and Hoogsteen hydrogen bonding are fundamental for the formation and stability of DNA motif structures. Hoogsteen hydrogen bonds typically appear in three-dimensional DNA structures, particularly within DNA helices. These bonds add an extra layer of stability by enabling alternative pairing configurations, making them crucial elements in the design of DNA-based hydrogels [67]. Hoogsteen triplex structures can be formed among abundant T-A sequences, C-G-C sequences, and C sequences [68]. The Hoogsteen hydrogen bonds between cytosine bases lead to the formation of i-motif quadrupleness [[69], [70], [71]]. DNA-based hydrogel systems can trigger the formation or breakage of hydrogen bonds under specific conditions, such as temperature or pH, thereby dynamically adjusting their physical or chemical properties [72]. The formation of the i-motif structure depends on acidic conditions [73]. When the pH value of the solution is increased to neutral or alkaline, the cytosine-cytosine pairing loses stability, leading to the disintegration of the i-motif structure. Liu ’s group reported an acid-resistant and physiological pH-responsive DNA-based hydrogel for oral insulin delivery (Fig. 2B) [74]. This hydrogel, incorporating A-motif and i-motif structures, was stable in extremely acidic conditions (pH as low as 1.2) and disintegrated at physiological pH levels, leading to release of the encapsulated insulin.
2.3. Versatility
ODNs are short sequences of single-stranded DNA, typically composed of about 8–50 deoxynucleotide units and can be chemically modified. ODNs can exhibit antioxidative and anti-inflammatory activities and alleviate local inflammation [[75], [76], [77], [78]]. A type of inhibitory ODNs contains repetitive TTAGGG sequences found in mammalian telomeres. They inhibit the production of Th1 and pro-inflammatory cytokines, suppressing innate immune activation for anti-inflammatory effects [79]. Another ODNs sequences contain methylated CG or unmethylated GC sequences, which selectively block immune activation [80]. Wang reported self-assembled nanocentipedes as multivalent vehicles for the delivery of immunostimulatory CpG ODNs (Fig. 2C) [81]. A long DNA scaffold was initially self-assembled using the hybridization chain reaction (HCR). This scaffold, containing multiple biotin molecules, was designed to bind biotinylated CpG ODNs via streptavidin-biotin interactions to create a stable nanostructure with immunostimulatory properties. The internalization of multivalent vehicles by RAW264.7 cells leaded to the secretion of substantial cytokine levels, effectively inducing cell apoptosis. DNA-based hydrogels, constructed with ODN, show promise in regulating immune responses at pathological sites.
Aptamers are short nucleotide sequences, typically 20 to 100 nucleotides long, composed of DNA [82]. Aptamers can fold into specific three-dimensional structures such as hairpins, internal loops, bulges, pseudoknots, G tetramers, or single-stranded loops, enabling them to recognize target molecules with high binding affinity [83,84]. Aptamers, obtained through “Systematic Evolution of Ligands by Exponential Enrichment (SELEX)” bind to target molecules with high affinity and specificity [83,85]. Aptamers that can target small metal ions, amino acids, organic molecules, proteins, viruses, and bacteria have been generated [86]. The binding between aptamers and their targets, mediated by van der Waals forces, hydrogen bonding, electrostatic interactions, stacking of flat moieties, and shape complementarity, is akin to antigen-antibody reactions [87]. Aptamers are also known as chemical antibodies. Rukhma et al. developed a novel hydrogel integrated the VEGF 165-specific aptamer. The aptamer could bind to VEGF165 and promote the slow release of vascular endothelial growth factor (VEGF). The local bioactivity of VEGF was increased through spatiotemporal selectivity (Fig. 2D) [88]. This approach enabled the flexible design of therapeutic strategies that can be precisely tailored to address the unique conditions of the bone microenvironment, offering a customized solution for bone regeneration.
3. DNA-based hydrogels construction and structure optimization
3.1. Construction of DNA-based hydrogels
DNA-based hydrogels can be classified into two categories: pure DNA-based hydrogels and hybrid DNA-based hydrogels. Pure DNA-based hydrogels can be constructed using methods such as enzymatic assembly or HCR [89]. Pure DNA-based hydrogels have lower mechanical strength, but this issue can be addressed by incorporating nanoparticles or synthetic polymers to create hybrid DNA-based hydrogels [90]. The hybrid DNA-based hydrogels enhance the mechanical properties while maintaining the functional versatility of the pure DNA-based hydrogel, making them more suitable for applications that require greater durability and stability [32,91]. We summarize the advantages and disadvantages of various hydrogel construction methods, highlighting their suitability for specific applications (Table 1).
Table 1.
Summary of DNA-based hydrogel construction and application.
| Construction | Method | Advantages | Disadvantages | Specific Application | Reference |
|---|---|---|---|---|---|
| Pure DNA-based hydrogels | Enzyme catalyzed reaction | Physiological conditions gelation Biocompatibility |
Low mechanical Strict reaction conditions Enzyme activity |
Drug release 3D cell culture |
[92] |
| Complementary base pairing | Enzyme-free Rapid gelation High sensitivity Simple and cost-effective Tunable physical properties |
Expensive DNase degradation Low mechanical properties |
Drug delivery Biosensors Diagnostic platforms |
[93] | |
| Hybrid DNA-based hydrogels | Complementary base pairing | Biodegradability pH-response | Low mechanical properties | Drug release Tissue engineering |
[[94], [95], [96], [97]] |
| Hoogsteen hydrogen bonding | Photophysical properties pH-response Electrostatic interaction |
Low mechanical properties Complex fabrication |
3D cell culture Drug delivery |
[96,98] | |
| Condensation reaction | Adjustable cross-linking | Soft | Forensic applications | [99] | |
| Click chemistry | Rapid gelation | Endonuclease degradation | Biomedicine | [100] |
3.1.1. Construction of pure DNA-based hydrogels
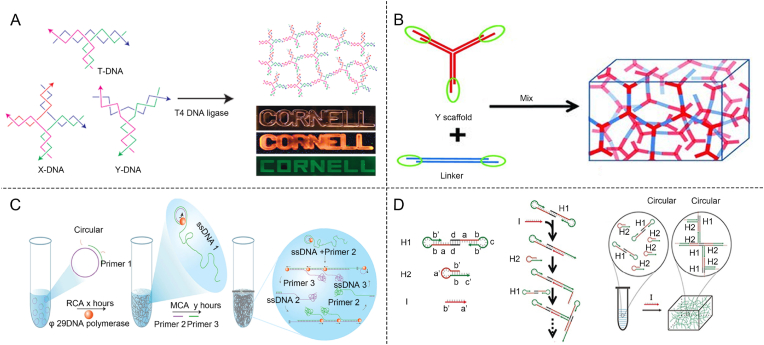
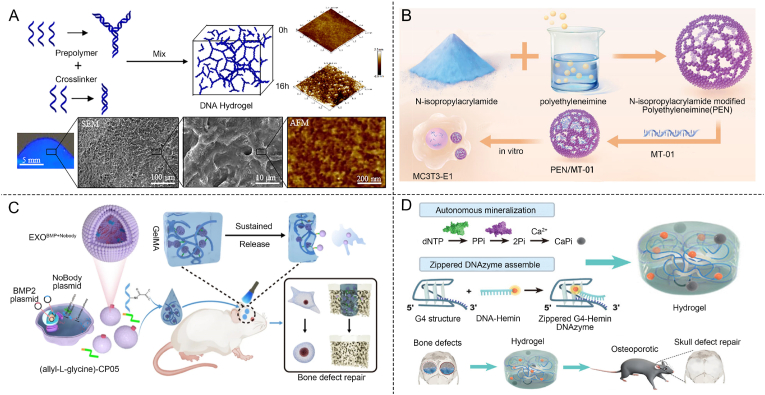
Pure DNA-based hydrogels can be synthesized by designing and chemically synthesizing a well-defined sequence of oligonucleotides through solid-phase synthesis. These oligonucleotides are then used to create distinct structural units, such as X-type, T-type, and Y-type, which form the building blocks of the DNA-based hydrogels [101]. The synthesized structural units serve as a crucial building block for the formation of the DNA-based hydrogel, which is achieved by enzymatic ligation or self-assembly through complementary base pairing [93]. Um et al. developed a method for enzyme-catalyzed assembly of pure DNA-based hydrogels by utilizing two ssDNA sequences. Through Watson-Crick base pairing, distinct structural units such as X-type, T-type, and Y-type were formed. These units were then covalently linked via phosphodiester bonds, catalyzed by T4 DNA ligase, enabling the construction of pure DNA-based hydrogels under physiological conditions through ligase-mediated reactions (Fig. 3A) [92]. In addition to enzymatic ligation, DNA-based hydrogels can be formed by utilizing complementary base pairing at the termini. This approach allows the simple physical mixing, leading to hydrogel formation. Liu et al. designed a self-assembling DNA-based hydrogel with programmable thermal and enzymatic responsiveness. The hydrogel was constructed using single-stranded DNA (ssDNA) to form Y scaffolds and L linkers. By mixing the two components, thermosensitive DNA-based hydrogels with tunable mechanical properties were created, driven by the complementary base pairing of the “sticky ends” (Fig. 3B) [93]. The specific DNA sequences of the “sticky ends” and the incorporated restriction sites endow the DNA-based hydrogels with enzymatic and thermal responsiveness, making them highly suitable materials for controlled drug release.
Fig. 3.
Construction method of pure DNA-based hydrogels. (A) Y X T shape DNA-based hydrogels formed by DNA ligase [92]. Copyright 2006, Reproduced with permission. (B) Y-scaffold and Linker base complementary pairing to form thermosensitive hydrogel [93]. Copyright 2011, Reproduced with permission. (C) Formation of DNA-based hydrogels using RCA amplification [102]. Copyright 2012, Reproduced with permission. (D) Synthesis of DNA-based hydrogels using HCR method [104]. Copyright 2017, Reproduced with permission.
Pure DNA-based hydrogels can also be constructed using Rolling Circle Amplification (RCA) and Hybridization Chain Reaction (HCR). Luo et al. constructed pure DNA-based hydrogels using RCA (Fig. 3C) [102]. RCA employs a circular single-stranded DNA template and φ29 DNA polymerase to produce ultra-long single-stranded DNA molecules with periodic and repetitive sequences. These extended DNA chains spontaneously form hydrogels through physical interactions, primarily driven by the entanglement and hybridization of the repetitive sequences [95,98]. This method offers distinct advantages, including high yield and sequence purity, as well as the ability to control the mechanical properties. Additionally, RCA-based DNA-based hydrogels can be functionalized with bioactive molecules, making them useful in various applications such as drug delivery, tissue engineering, and biosensing. Unlike RCA, HCR represents an alternative approach for constructing pure DNA-based hydrogels. HCR is an isothermal nucleic acid amplification method without the use of enzymes [103]. Wang et al. reported this technique by developing HCR-based approaches for the self-assembly of patterned DNA-based hydrogels, demonstrating its potential in constructing complex nanostructures with precise control. Triggered by specific conditions such as temperature and ion concentration, HCR proceeded spontaneously, driven by the complementary pairing of primers. This design allowed for a controlled sol-gel transition initiated by a targeted DNA sequence, ultimately leading to the formation of pure DNA-based hydrogels (Fig. 3D) [104].
3.1.2. Construction of hybrid DNA-based hydrogels
Hybrid DNA-based hydrogels can be synthesized by cross-linking copolymers composed of hydrophilic polymer chains, such as polyacrylamides and polypeptides, with modified DNA sequences [95,105]. These hydrogels can be further modified by incorporating materials like gold or silver nanoclusters, graphene, carbon nanotubes, magnetic nanoparticles, or clay, creating versatile structures with enhanced properties [[106], [107], [108], [109], [110]]. This modification enhances the desirable optical, electronic, and electromagnetic properties of hydrogels. By modifying DNA and polymerizing it with various polymers, researchers can create hybrid DNA-based hydrogels with tailored properties, offering promising applications in drug delivery. Willner et al. reported a series of functional DNA-hybrid hydrogels based on polyacrylamide chains [94,111]. In the synthesis of DNA-based hydrogels, acrydite-modified nucleic acids were first prepared, which were then copolymerized with acrylamide to form the hydrogel network. The acrydite groups enabled covalent bonding between the nucleic acids and acrylamide chains, resulting in a stable hydrogel structure. This system featured a DNA-based shape-memory hydrogel capable of transitioning between gel and liquid states. In this innovative design, long DNA strands woven by polymerase facilitate dynamic behavior. Notably, the hydrogel dissolved into a solution at pH 8.0 and reassembled into a gel at pH 5.0, allowing for reversible transitions between solution and gel states through pH cycling (Fig. 4A) [94].
Fig. 4.
Construction method of hybrid DNA-based hydrogels. (A) DNA-based hydrogels with pH-responsive acrylamide functionalization [94]. Copyright 2015, Reproduced with permission. (B) DNA-based hydrogels constructed from silica and carbon nanotubes [98]. Copyright 2019, Reproduced with permission. (C) Carbon dots combined with DNA to construct hydrogels [96]. Copyright 2017, Reproduced with permission. (D) Using nanoparticles to construct magnetically responsive DNA-responsive hydrogels [97]. Copyright 2020, Reproduced with permission.
In addition to polymers, DNA hybrid hydrogels formed by modified DNA with nanoparticles warrant significant attention. DNA-modified silica nanoparticles (SiNPs) offer distinct advantages, including excellent biomimetics and ease of synthesis. Hu et al. developed a novel DNA nanocomposite by polymerizing and interweaving SiNPs, carbon nanotubes, and DNA (Fig. 4B) [98]. The synthesis of DNA-based hydrogels through SiNP/CNT-DNA nanocomposites involved functionalizing SiNPs with aminoalkyl-modified ssDNA using glutaraldehyde cross-linking and wrapping carbon nanotubes (CNTs) with ssDNA via π-π stacking interactions. These functionalized SiNPs and CNTs were then used as primers in RCA process, leading to the formation of SiNP/CNT-DNA nanocomposites. This innovative strategy enabled the creation of DNA-based hydrogels with tunable mechanical properties and viscosity, achieved through the integration of inorganic components. These versatile materials functioned as matrices with enhanced control over cell adhesion, proliferation, and migration.
Carbon dots, a well-established class of nanoparticles, are valued for their water solubility, biomimetics, and cost-effectiveness [112]. Singh et al. developed a hybrid hydrogel composed of DNA and carbon dots for targeted and sustained release of therapeutic agents (Fig. 4C) [96]. The amine-functionalized water-soluble carbon dots were conjugated to 5′-phosphorylated ssDNA. The coupling agents activated the terminal phosphate group of the ssDNA, which was then attached to the CDs. For hydrogel formation, the DNA sequence was designed with a C-rich region to facilitate the formation of an i-motif structure under slightly acidic conditions. The chemotherapy drug doxorubicin (Dox) was encapsulated within the hydrogel. The hydrogel demonstrated prolonged release of Dox in acidic environments, highlighting its potential for drug delivery.
Hybrid DNA-based hydrogels constructed with magnetic nanoparticles possess the unique ability to change shape and provide mechanical stimulation when guided by an external magnetic field. Yang et al. developed a magnetic DNA hybrid hydrogel (Fig. 6D) [97]. The DNA-based hydrogel synthesis involves RCA using a circular DNA template to produce ultra-long ssDNA. This ssDNA contains specific capture sequences designed to hybridize with short ssDNA-modified magnetic nanoparticles (MNPs), forming permanent crosslinks. Further stability is provided through secondary amplification using the ultra-long ssDNA as a template and dynamic crosslinking from ssDNA entanglement. DNA-based hydrogel-based nanorobots can undergo responsive deformation in response to magnetic field strength changes. These hydrogels can move under driven by magnetic stimuli. The porous structure enables them to serve as intelligent vehicles for drug or cell delivery [[113], [114], [115]].
Fig. 6.
The mechanism of DNA-based hydrogels promoting bone. (A) Degradation of DNA-based hydrogels release phosphate to promote mineralization and osteogenesis [143]. Copyright 2023, Reproduced with permission. (B) ODNs promotes osteogenic differentiation of BMSCs, which is beneficial for osteogenesis [153]. Copyright 2023, Reproduced with permission. (C) Delivery of BMP-2 promotes bone regeneration [162]. Copyright 2023, Reproduced with permission. (D) DNA-based hydrogels reduce ROS in the bone microenvironment and promotes bone regeneration [147]. Copyright 2021, Reproduced with permission.
3.2. Structure optimization
Bone tissue is characterized by a sophisticated three-dimensional architecture. In bone tissue engineering, the spatial structure is crucial for supporting bone regeneration. Several methods have been developed to enhance the structure and versatility of DNA-based hydrogels, including bulk hydrogel fabricating, microsphere formatting, and 3D printing techniques [116]. Bulk hydrogels play a crucial role in tissue engineering by providing structural support and protection for cells. Hydrogel microspheres provide a high surface area for cell attachment and nutrient exchange. 3D printing allows for the precise construction of complex and biomimetic bone structures. Bulk hydrogels and hydrogel microspheres often struggle to accurately replicate the complex microstructure of bone tissue [117]. Although 3D printing offers the ability to construct intricate structures, it faces limitations in the selection of suitable bio-inks [118].
3.2.1. Bulk hydrogels fabricating
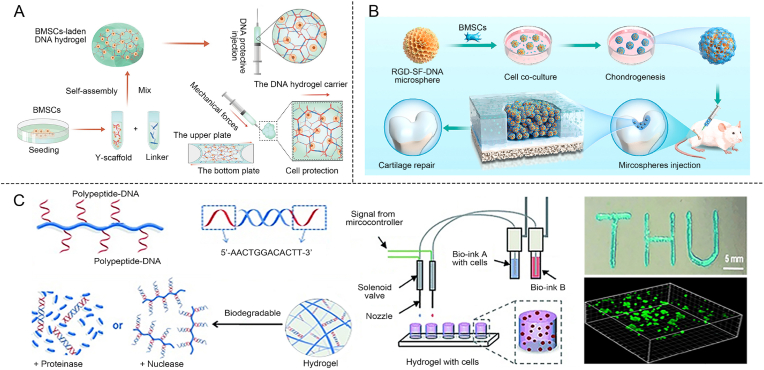
The bulk hydrogels serve as a supportive framework for the growth and differentiation of bone cells, with cells implanted to special structure. Ensuring cell viability and versatility during and after encapsulation is critical for successful tissue integration and therapeutic outcomes. Yu et al. studied the anti-shear protection provided by DNA supramolecular hydrogels loaded with BMSCs in vitro and in vivo (Fig. 5A) [119]. When the hydrogel precursor solutions containing BMSCs are mixed, a hydrogel is rapidly formed within seconds through terminal base-pairing complementarity. This quick gelation process leveraged the complementary base pairing of the DNA strands to create a stable hydrogel structure almost instantly. Due to the dynamic nature of supramolecular interactions, a lubricating layer formed inside the syringe during injection, reducing shear forces caused by velocity differences and thereby enhancing cell survival rates [120]. This approach supported both cell survival and tissue integration, making it a powerful strategy in bone tissue engineering. However, bulk hydrogels, being large, solid 3D cell culture matrices, are often too simplistic and cannot meet the precise, fine-tuned requirements needed for more advanced tissue engineering applications.
Fig. 5.
Construction of bone organoids with advanced structure of DNA-based hydrogels. (A) Using DNA supramolecular hydrogel to encapsulate BMSC to construct a shear-resistant cell delivery system [119]. Copyright 2021, Reproduced with permission. (B) DNA-based hydrogel microspheres to construct cartilage organoids [127]. Copyright 2024, Reproduced with permission. (C) Use peptides and DNA to construct new bioinks and perform cell-laden 3D bioprinting to build multi-layer structures [22]. Copyright 2015, Reproduced with permission.
3.2.2. Microspheres formatting
Compared to bulk hydrogels, hydrogel microspheres offer several advantages due to their higher specific surface area, which significantly enhances cell adhesion and proliferation [121,122]. Additionally, their increased porosity allows for more efficient transport of nutrients and waste, thereby improving the exchange of essential substances [123,124]. This enhanced transport capability helps maintain cell viability and supports tissue regeneration around the hydrogel microspheres, making them highly effective in biomedical applications [125,126]. Using microfluidic technology, the size and characteristics of microspheres can be precisely controlled, enabling the rapid and efficient synthesis of large quantities of hydrogel microspheres with uniform particle sizes. Su et al. constructed RGD-SF-DNA-based hydrogel microspheres (RSD-MSs) by utilizing SilMA (Fig. 5B) [127]. Using microfluidic technology, hydrogel microspheres were prepared by precisely controlling the mixing of the hydrogel precursor solution with the oil phase within microchannels, forming uniform droplets. These RSD-MSs significantly enhanced the proliferation, adhesion, and chondrogenic differentiation of BMSCs. Transcriptomic analysis revealed that RSD-MSs promoted chondrogenesis primarily via integrin-mediated adhesion pathways and glycosaminoglycan biosynthesis. Moreover, seeding BMSCs onto RSD-MSs to create cartilage organoid precursors (COPs) markedly improved cartilage regeneration, highlighting the potential of this approach for advanced tissue engineering applications.
3.2.3. 3D printing
3D printing, a cutting-edge manufacturing technique for designing and fabricating tissue-like structures, has garnered considerable attention in tissue engineering [128,129]. 3D bioprinting technology includes several key methods for fabricating complex tissue structures [130,131]. These methods include extrusion-based bioprinting, where bioink is deposited layer by layer, and digital light processing (DLP), which offers faster printing with high accuracy at the micron scale [[132], [133], [134]]. The selection of each method depends on the specific requirements of the tissue being printed, such as the material properties, resolution, and cell types needed. One of the key challenges in 3D bioprinting is selecting the appropriate hydrogel material to serve as bioink for constructing the extracellular matrix [135]. Liu et al. reported the first method of using DNA-based hydrogels as bioinks for rapid in situ multi-layer 3D bioprinting. Using an extrusion 3D printer, peptide-DNA conjugates (Bioink A) were combined with complementary DNA linkers (Bioink B), causing the mixture to solidify within seconds (Fig. 5C) [22]. Liu et al. successfully printed multi-layer structures such as “THU” and triangles using this method. Additionally, cells were mixed with the bioinks and printed, and after 48 h, live/dead staining demonstrated cell viability rates of over 99 %. This highlighted the potential of DNA-based hydrogels for 3D bioprinting. The combination of DNA-based hydrogels, 3D printing technology, and the high survival rate of BMSCs opens new possibilities for constructing spatially biomimetic bone extracellular matrices in the future.
4. Bone regeneration using DNA-based hydrogels
DNA-based hydrogels serve as excellent matrix materials. Compared to other hydrogels such as PEG-based, gelatin methacryloyl (GelMA), hyaluronic acid methacrylate (HAMA), chitosan, or alginate hydrogels, DNA-based hydrogels possess unique advantages due to their inherent characteristics [136]. As carriers of genetic material, DNA-based hydrogels are biomimetics, programmability and versatility allowing precise control over their structure and function, unlike traditional hydrogels that are often more static in nature. These versatile characteristics make DNA-based hydrogels a powerful tool in bone regeneration by promoting osteogenesis, angiogenesis, and immune modulation (Table 2). Their ability to precisely control cellular processes and dynamically respond to environmental cues positions them as an essential material in tissue engineering and regenerative medicine.
Table 2.
Summary of DNA-based hydrogels for promoting bone regeneration.
| Strategy | Type | Existence | Function | Reference |
|---|---|---|---|---|
| Osteogenesis | Y scaffold and L linker | Pure DNA-based hydrogel | Phosphate ions produced by degradation combine with calcium ions to form HAP, promoting mineralization. | [143] |
| Plasmids | GelMA Encapsulation | Plasmid delivers BMP-2 gene to promote local expression of BMP-2 and promote osteogenesis. | [162] | |
| Aptamers | Michael Addition reaction with PEG | Targeted recruitment of BMSCs and promotion of bone differentiation. | [163] | |
| DNAzyme | Pure DNA-based hydrogel by RCA | Eliminate ROS, improve diabetic bone microenvironment and promote osteogenesis. | [147] | |
| Angiogenesis | CRISPRa system | Hyaluronic Encapsulation | Gene delivery promotes VEGF-A expression and angiogenesis | [167]. |
| ss DNA tFNAs |
Complementary base pairing tFNA delivery of miRNA | tFNA delivery of AP02 promotes angiogenesis. | [169] | |
| Aptamer | Fibrin covalently bound | Targeting VEGF to promote angiogenesis. | [170] | |
| Aptamer | Complementary base pairing | Intelligent response degradation of MMP-9 enzyme specific to the diabetic microenvironment. | [171] | |
| Immuno-modulation | Salmon sperm DNA | DNA and Collagen are covalently bound | Milder immune response by regulating T cells. | [16] |
| Y scaffold and L linker | Pure DNA-based hydrogel | Release of exosomes promotes macrophage polarization to M2 and promotes tissue repair. | [181] | |
| Y scaffold and Linker Sliver nanoparticles |
Pure DNA-based hydrogel | DNA combined with silver nanoparticles to promote antibacterial function. | [184] |
4.1. Osteogenesis-based bone regeneration
DNA-based hydrogels are powerful tools for promoting bone regeneration, offering a multi-faceted approach to tissue engineering [137]. They release phosphate ions, which contributes to the mineralization process crucial for bone formation. Additionally, the incorporation of tFNA enhances osteogenic differentiation. DNA-based hydrogels can also be functionalized with aptamers that target BMSCs, ensuring precise delivery and interaction at the bone regeneration site [138]. Furthermore, DNAzymes within the hydrogel act as catalysts to facilitate various biochemical processes to accelerate bone regeneration.
4.1.1. Phosphate ions
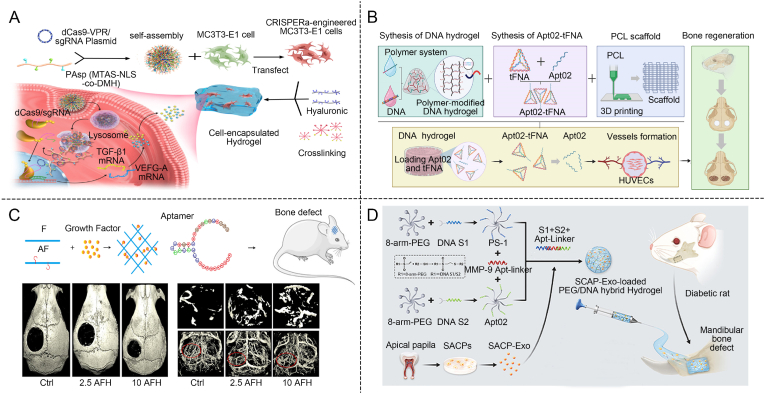
DNA-based hydrogels can mimic the natural processes involved in bone formation, highlighting their potential as a valuable tool in tissue engineering and regenerative medicine [[139], [140], [141], [142]]. As DNA degrades, it releases phosphate ions into the surrounding environment. These phosphate ions exhibit a strong affinity for calcium ions (Ca2+), leading to the formation of calcium phosphate (CaP), which subsequently converts into hydroxyapatite (HAP). The HAP crystals align along the DNA backbone, facilitating bone tissue regeneration by mimicking the natural mineralization process of bone [143,144]. Notably, extracellular phosphate can promote the osteogenic differentiation and mineralization of pre-osteoblasts and mesenchymal stem cells. Additionally, extracellular adenine, a byproduct of DNA degradation, has been demonstrated to act as a promoter of osteogenic differentiation. Similarly, RCA-mediated DNA-based hydrogel formation also generates phosphate ions as byproducts, further promoting mineralization [145,146]. These combined processes enhance the mineralization potential of DNA-based materials, making them more effective in bone tissue engineering applications [147].Carneiro et al. utilized DNA-based hydrogels constructed with Y scaffolds and crosslinkers to study the interactions between the hydrogels and osteoblastic cell lines MC3T3-E1 and mouse cranial osteoblasts, both in vitro and in vivo (Fig. 6A) [143]. Atomic force microscopy revealed a significant increase in 3D spatial hydroxyapatite formation just 16 h after the implantation of the DNA-based hydrogels. Furthermore, when implanted into cranial defect animal models, the DNA-based hydrogels induced substantial new bone formation in critical-sized cranial defects in rats after four weeks. This ability to mimic natural bone formation processes underscores the potential of DNA-based hydrogels as a valuable tool in tissue engineering and regenerative medicine.
4.1.2. ODNs
Specific ODNs have a effect on the regulation of bone regeneration [148]. These ODNs play a key role in modulating bone remodeling by influencing both bone-resorbing osteoclasts and bone-forming osteoblasts, making them valuable tools in understanding and potentially treating bone-related diseases [149,150]. MT01, a synthetic 27-mer single-stranded ODN based on human mitochondrial DNA, enhances the maturation and activation of rat osteoblasts. It has been shown to reduce alveolar bone resorption caused by periodontitis in rats and to regulate the expression of key bone formation-related factors such as Runx-2, collagen type I (COL I), and osteoprotegerin (OPG). Additionally, MT01 promotes the differentiation of BMSCs into osteoblasts, playing a crucial role in bone remodeling [151,152]. Due to the susceptibility of free ODNs to degradation by nucleases in serum, extensive research has focused on chemically modifying ODNs and integrating them with various materials to enhance their stability and functionality. The Wang group utilized a Michael addition reaction to modify polyethyleneimine (PEI) with N-isopropylacrylamide, resulting in a PEI derivative (Fig. 6B). This N-isopropylacrylamide-modified polyethyleneimine (PEN) was then used to deliver the ODN MT01 in models of MC3T3-E1 cells and rat cranial bone defects, achieving promising results in bone defect treatment [153]. The PEN/MT01 treatment group exhibited a significant increase in alkaline phosphatase (ALP) enzyme activity, demonstrating strong osteogenic effects and highlighting the potential of this approach for bone regeneration.
4.1.3. tFNA
Tetrahedral Framework Nucleic Acid (tFNA) structures are a widely utilized form of TDN. Their unique three-dimensional architecture allows them to efficiently encapsulate and deliver these molecules to target cells [154,155]. MicroRNAs (miRNAs) play a crucial role in regulating gene expression within cells by binding to target mRNA, leading to mRNA degradation or inhibition of its translation [156]. miRNAs must enter cells and reach the cytoplasm to exert their effects. Using tFNAs as carriers for miRNA delivery into cells can help maintain both the transport and stability of miRNA within the body, offering promising new research opportunities for bone regeneration. Cai et al. utilized RNase H-responsive sequence-linked sticky-end tFNA (stFNA) and miR-2861 to specifically target the expression of histone deacetylase 5 (HDAC5) in BMSCs [157]. This bioswitchable nanocomposite (stFNA-miR) effectively upregulated miR-2861 expression after intracellular delivery, leading to the inhibition of HDAC5 protein expression. Alizarin red and ALP staining confirmed that the tFNA have shown significant osteogenic activity. This highlights the potential of tFNA-based delivery systems in promoting bone formation.
4.1.4. Plasmid
Plasmid DNA (pDNA) delivery into cells is a powerful tool for gene therapy and biomedical research. Once inside the cell, pDNA can be transcribed to produce therapeutic proteins or enzymes, which can correct genetic defects, combat diseases, or influence cellular behavior. Unlike transient RNA-based approaches, pDNA can offer sustained expression of the encoded genes, making it valuable for long-term therapeutic strategies. Osteoinductive growth factors play a crucial role in bone healing by facilitating the recruitment, proliferation, and osteogenic differentiation of BMSCs [158,159]. Among these growth factors, bone morphogenetic protein-2 (BMP-2) represent the growth factors most thoroughly researched within the field of bone tissue engineering [160]. BMP-2 upregulates transcription factors like Osterix or Runx2, promoting the osteogenic differentiation of BMSCs [161]. Using hydrogels to deliver BMP-2 plasmid DNA enables sustained gene expression, which promotes osteogenic differentiation and enhances bone repair. This method allows for the continuous production of BMP-2 at the treatment site, ensuring a prolonged effect on bone regeneration. Wang et al. developed a DNA-based hydrogel system for the localized delivery of pDNA to the site of bone defects. This hydrogel enables the endogenous cell transcription machinery to continuously produce BMP-2 in situ (Fig. 6C) [162]. Engineered exosomes rich in BMP-2 mRNA were obtained by co-transfecting 293T cells with non-annotated P-body dissociating polypeptide (NoBody) and an artificial plasmid encoding BMP-2. Allyl-L-glycine-modified CP05, covalently bonded with GelMA, was employed as both a delivery system and a scaffold for the engineered exosomes, facilitating their sustained release. This DNA-based hydrogel represents a long-term, safe approach for bone regeneration.
4.1.5. Aptamer
Aptamer-functionalized hydrogels can specifically target BMSCs to enhance bone regeneration. By concentrating BMSCs at the injury site, aptamer-functionalized hydrogels not only improve cell delivery but also stimulate osteogenic differentiation, accelerating bone repair. Du et al. construct aptamer-functionalized hydrogels which promote bone healing by selectively recruiting endogenous BMSCs [163]. The functional hydrogels were synthesized by reacting maleimide-terminated 4-arm PEG with a thiol-flanked PEG crosslinker, enabling rapid in situ gel formation. To achieve high-density aptamer coverage on the hydrogel surface, an aldehyde group-modified BMSC-specific aptamer was covalently bonded to the thiol-flanked PEG crosslinker. This approach ensures efficient aptamer integration, enhancing the hydrogel's ability to interact with targeted cells and support tissue regeneration. These BMSC-aptgels effectively recruited BMSCs and maintained their activity both in vitro and in vivo. By regulating osteogenesis and inflammation, BMSC-aptgels significantly accelerated bone healing, highlighting their potential in regenerative medicine.
4.1.6. DNAzyme
In DNA-based hydrogel construction, DNA chains can form DNAzyme. These DNA molecules possess catalytic activity and can drive various chemical reactions. Unlike traditional protein-based enzymes, DNAzymes are composed entirely of nucleotides but can still exhibit remarkable catalytic efficiency and specificity under appropriate conditions. DNAzymes play a crucial role in the bone microenvironment by modulating key factors such as pH, ion concentration, and biomolecule interactions. Wu et al. have developed a novel DNA-based hydrogel that offers an innovative approach to enhancing osteoporotic bone regeneration (Fig. 6D) [147]. By designing DNAzyme sequences within the RCA process, large quantities of DNA with enzymatic functions can be generated, resulting in DNA-based hydrogels with embedded DNAzyme activity. Increased ROS levels are a hallmark of osteoporotic bone tissue. Elevated ROS levels can alter the differentiation pathway of BMSCs from osteogenesis towards adipogenesis [164]. This condition negatively affects osteoblast functions. MDH incorporates G4-Hemin DNAzyme within the DNA-based hydrogel, designed to scavenge ROS and create a favorable environment for bone repair. The study demonstrated that as the DNA-based hydrogel degraded and G4-Hemin DNAzyme was released, ROS levels in the bone microenvironment were significantly reduced. The hydrogel exhibited excellent ability to enhance osteoblast activity and support bone tissue regeneration under osteoporotic conditions, both in vitro and in vivo.
4.2. Angiogenesis-promoted bone regeneration
Vascularization is essential for successful bone regeneration. Incorporating the CRISPRa system, tFNA, and aptamers into DNA-based hydrogels provides a powerful approach to promoting angiogenesis, a key process in bone healing [165,166]. These technologies offer precise and targeted approaches to enhance angiogenesis, supporting more effective bone regeneration.
4.2.1. Gene
Introducing the CRISPRa system into hydrogels represents a cutting-edge strategy to enhance tissue regeneration, particularly in bone healing. CRISPRa is a modified version of the CRISPR-Cas9 system that activates gene expression. The non-viral CRISPRa system offers several advantages, including high safety, low immunogenicity, and increased genetic payload capacity. By embedding this system within hydrogels, it becomes possible to locally stimulate the expression of key angiogenic and osteogenic genes at the site of injury or bone defect. This leads to sustained production of growth factors such as VEGF-A for angiogenesis. The localized gene activation provided by CRISPRa within the hydrogel creates a bioactive environment that promotes faster and more effective vascularization and bone regeneration. The Chen group developed a tunable dual-crosslinked DNA-based hydrogel (Fig. 7A) [167]. This hydrogel system utilized a cationic polymer equipped with nucleus-localizing peptides and proton sponge groups to deliver the CRISPRa system. Osteogenesis was enhanced by overexpressing TGF-β1 and VEGF-A genes in preosteoblast MC3T3-E1 cells. The results showed that mice treated with the combined activation of these genes exhibited the highest new bone volume at all time points, with osteogenesis increased by 4.5-fold and 2.5-fold. These findings demonstrated that the combined activation of TGF-β1 and VEGF-A via non-viral CRISPRa systems significantly enhances the osteogenic potential of preosteoblasts encapsulated in dual-crosslinked hydrogels, offering a promising approach to bone tissue regeneration.
Fig. 7.
DNA-based hydrogels promote bone through vascularization. (A) Dual-crosslinked hydrogel deliver CRISPRa system to overexpress promote vascularization [167]. Copyright 2022, Reproduced with permission. (B) Polymer DNA-based hydrogel incorporates tFNA to promote vascularization and promote bone regeneration [169]. Copyright 2024, Reproduced with permission. (C) Aptamer modification of DNA-based hydrogel targeting VEGF promotes vascularized bone repair [170]. Copyright 2019, Reproduced with permission. (D) MMP-9 responds to Diabetes mellitus pathological state to promote vascularization for bone regeneration [171]. Copyright 2022, Reproduced with permission.
4.2.2. tFNA
tFNA inherently possess angiogenic properties, making them valuable in promoting vascularization. Due to their unique structure, tFNAs can interact with endothelial cells, stimulating the formation of new blood vessels [168]. Su's group developed a polymer-modified DNA-based hydrogel using the latest nucleic acid nanotechnology (Fig. 7B) [169]. They used acrydite-modified DNA to copolymerize with acrylamide, forming a polymer. The hydrogel was then created at 37 °C through base-pairing interactions between the single-stranded DNA, resulting in a stable gel structure. The presence of nucleases in the body can degrade DNA, compromising the long-term bone repair function of DNA-based hydrogels. Actin is added to the hydrogel to inhibit enzymatic degradation, thereby extending the stability of the DNA-based hydrogel. SEM imaging revealed that the DNA-based hydrogel possesses a porous structure. Additionally, tFNA was incorporated into the DNA-based hydrogel to enhance osteogenic mineralization, with the tFNA structure clearly visible under TEM. tFNA was further modified with Apt02, which has angiogenic properties. This dual approach significantly accelerated both the osteogenic differentiation of BMSCs and the angiogenesis of human umbilical vein endothelial cells (HUVECs). In vivo studies on rats with critical-size cranial defects demonstrated the hydrogel's efficacy in promoting new bone formation.
4.2.3. Aptamer
VEGF aptamers can be engineered to enhance VEGF activity by stabilizing the growth factor, prolonging its presence in the bloodstream, or modifying its interaction with receptors to more effectively promote angiogenesis. Donahue et al. developed an aptamer-functionalized fibrin hydrogel (Fig. 7C) [170]. The capability of aptamer functionalization for in situ injection into fibrin hydrogels, enabling the delivery of VEGF factors in vivo, was investigated. The aptamer-functionalized fibrin hydrogel retained more VEGF compared to natural fibrin hydrogels and released these growth factors continuously, exhibiting higher biological activity. Injecting aptamer-functionalized fibrin hydrogel into bone defects in mice enables the simultaneous delivery of VEGF, promoted the formation of mature blood vessels, and further supports bone tissue regeneration.
In the pathological state of diabetes mellitus (DM), elevated oxidative stress and metabolic dysregulation promote the overexpression of proinflammatory markers, such as matrix metalloproteinases (MMPs). To construct an MMP-9-responsive hydrogel crosslinker, aptamers can also be designed to specifically bind to the MMP-9 enzyme. These aptamer-based crosslinkers are incorporated into the hydrogel, and upon exposure to MMP-9, the enzyme cleaves the crosslinker, triggering controlled degradation of the hydrogel. This allows for targeted release of therapeutic agents or other bioactive molecules in environments where MMP-9 is active. Song's group developed a biocompatible polyethylene glycol (PEG)/DNA hybrid hydrogel that releases exosomes in diabetic microenvironment to promote angiogenesis for treating diabetic bone defects (Fig. 7D) [171]. An MMP-9-responsive aptamer sequence was designed to enable the targeted degradation of the hydrogel. SCAP-Exo demonstrated strong abilities to promote vascularization and bone formation in vitro, under both normal and diabetic conditions. Western blot analysis of the exosomes revealed upregulated expression of angiogenic proteins. Notably, the HUVEC tube formation assay showed significantly enhanced tube formation in the PEG/DNA hybrid hydrogel group. When the injectable PEG/DNA hybrid hydrogel loaded with SCAP-Exo was injected into mandibular bone defects in diabetic rats, it resulted in a beneficial therapeutic effect, promoting both angiogenesis and bone regeneration.
4.3. Immunomodulation-promoted bone regeneration
The concept of “osteoimmunology” was first proposed by Arron and Choi [172]. As osteoimmunology advances, bone regeneration is understood to be not just a simple process involving bone formation and resorption but also the immune system [173]. Chronic conditions like osteoporosis, osteoarthritis, and bone defects are greatly related to changes in the bone tissue immune microenvironment. The transition from a pro-inflammatory to an anti-inflammatory environment is essential for supporting bone regeneration [174]. Immune regulation plays a crucial role in bone regeneration by modulating immune cell responses, particularly the polarization of macrophages, to coordinate inflammation and repair [175]. In the early stages of bone injury, macrophages exhibit an M1 phenotype, promoting inflammation to clear necrotic tissue and prevent infection. During the repair phase, macrophages transition to an M2 phenotype, exerting anti-inflammatory effects and facilitating angiogenesis, cell proliferation, and osteogenic differentiation [176]. T cells also play a crucial role in bone regeneration by regulating immune responses and promoting repair. In the early stages, CD4+ and CD8+ T cells secrete pro-inflammatory cytokines to aid in pathogen clearance; however, excessive inflammation may impair bone healing. As the repair phase progresses, Th2 cells and regulatory T cells (Tregs) secrete anti-inflammatory cytokines to promote osteogenic differentiation and bone healing [177]. Proper immune responses not only aid tissue repair but also enhance bone formation and healing, whereas excessive inflammation can hinder the regenerative process. Therefore, precise immune modulation is essential for successful bone regeneration. DNA have shown significant potential in immunomodulation. This immunomodulate ability is particularly important for bone regeneration.
4.3.1. tFNA
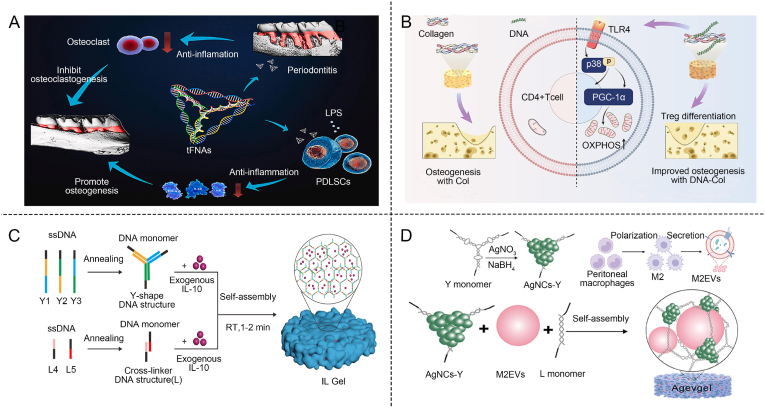
tFNA not only promotes angiogenesis but also possesses anti-inflammatory properties. It has been shown to stimulate the proliferation and osteogenic/odontogenic differentiation of dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLSCs) [178]. Lin's group designed and constructed tFNA to investigate whether it could suppress the release of inflammatory cytokines under both in vitro and in vivo inflammatory conditions. tFNA shows significant potential in modulating inflammation while simultaneously enhancing tissue repair and regeneration (Fig. 8A) [179]. Research has shown that tFNA reduces the release of pro-inflammatory factors and cellular ROS production, thereby enhancing the migration and osteogenic differentiation of PDLSCs in vitro. tFNA protects the osteogenic differentiation of PDLSCs under inflammatory conditions by inhibiting the MAPK/ERK signaling pathway. In a rat model of periodontitis, tFNA significantly decreased inflammatory cell infiltration and downregulated the expression of IL-6 and IL-1β. Through its regulatory functions, tFNA not only addressed the underlying immune responses associated with periodontal disease but also promoted the recovery and restoration of bone tissue.
Fig. 8.
DNA-based hydrogels regulate immunity and promotes bone regeneration. (A) DNA promotes bone regeneration in the periodontitis environment through anti-inflammatory effects [179]. Copyright 2021, Reproduced with permission. (B) DNA-Col scaffold regulating Treg cells to promote bone regeneration [16]. Copyright 2023, Reproduced with permission. (C) DNA-based hydrogel releases IL-10 to promote bone regeneration in diabetes [181]. Copyright 2022, Reproduced with permission. (D) AgNCs M2EVs DNA-based hydrogel regulates macrophage polarization and promotes bone regeneration [184]. Copyright 2023, Reproduced with permission.
4.3.2. DNA
By harnessing the immune-modulatory properties of DNA-based hydrogels, the activation and regulation of T helper (Th) cells can be directed to create a more favorable environment for bone regeneration [180]. T cells play a regulatory and amplifying role in the immune system and have a significant impact on bone regeneration. DNA-based hydrogels can be used to modulate Th cells, inducing effective immune responses that support bone healing and tissue repair. Niu et al. synthesized a biological scaffold formed by crosslinking deoxyribonucleic acid with collagen (DNA-Col) to promote bone repair (Fig. 8B) [16]. Immunofluorescence staining of the DNA-Col scaffold revealed that DNA successfully bound to the collagen matrix and led to an increase in CD4, Foxp3, and a significant rise in regulatory T cells (Tregs). The DNA-Col scaffold effectively promoted the recruitment and differentiation of Tregs, thereby improving the inflammatory microenvironment of bone tissue and exhibiting immunoregulatory functions. When implanted in a rat alveolar bone defect model, the DNA-Col scaffold played a significant role in new bone formation. Further research demonstrated the scaffold's impact on Treg differentiation, establishing DNA-Col as a bioactive bone regeneration scaffold through its interaction with Tregs. The use of DNA-based hydrogels can enhance tissue integration and regeneration by modulating immune responses.
The Y scaffold and linker DNA-based hydrogel play a crucial role in immunomodulating the bone microenvironment. Zheng's group developed a biodegradable, anti-inflammatory, and osteogenic IL DNA-based hydrogel (Fig. 8C) [181]. As the DNA-based hydrogel degrades, the released IL-10 regulated the surrounding immune microenvironment for facilitating bone repair. The porous microstructure of the IL gel was ideal for encapsulating IL-10, allowing for the sustained release of biologically active IL-10. This process not only promoted the polarization of M2 macrophages by reducing inflammation and alleviating periodontitis, but also triggered the osteogenic activity of BMSCs, accelerating the formation of alveolar bone. This dual action underscored the potential of IL DNA-based hydrogels in both immunomodulation and bone regeneration.
The Y scaffold constructed from DNA can be combined with silver nanoclusters (AgNCs) to form a DNA-based hydrogel. This hydrogel exhibits both the structural benefits of DNA scaffolding and the antimicrobial properties of AgNCs [182]. Various biomaterials loaded with AgNCs demonstrated satisfying antibacterial and immunomodulatory effects [183]. Tong's group developed an immunomodulatory DNA-based hydrogel, termed Agev gel, by integrating AgNCs and M2EVs into the DNA-based hydrogel (Fig. 8D) [184]. AgNCs were synthesized using DNA scaffolds as templates, serving as the antibacterial building blocks of the Agev gel, while immunomodulatory M2EVs are encapsulated within the shape-adaptive DNA scaffolds. The biodegradable, slow-release, antibacterial, and immunomodulatory properties of Agev gel work synergistically to reduce inflammation, promote cell proliferation, induce osteogenesis, and provide long-term prevention of bacterial infections. This combination ultimately enhanced bone regeneration in diabetic alveolar bone defects (DABD).
5. DNA-based hydrogels for promising bone organoids
DNA-based hydrogels are emerged as a potential material for bone regeneration due to their unique properties, including functionality, programmability, and biomimetic characteristics. As naturally occurring molecules, DNA-based hydrogels can be designed to replicate the structure and function of the extracellular matrix, providing a supportive environment for bone tissue regeneration. Dynamic bionic materials are of great significance for the construction of bone organoids. DNA-based hydrogels can be functionalized with bioactive molecules making them adaptable to the complex microenvironment of bone tissue. DNA-based hydrogels can facilitate osteogenesis, vascularization, and immune modulation, which are critical for developing effective bone organoids.
A variety of hydrogels are currently being utilized for the construction of bone organoids [185,186]. Matrigel has played a crucial role in the construction and application of organoids. However, since Matrigel is derived from mouse tumor tissue, it has a complex composition, significant batch-to-batch variability, and potential biosafety concerns due to its tumor origin. In recent years, GelMA, collagen and fibrin has gained attention as an important matrix material, owing to its rapid crosslinking properties and ease of preparation in the lab, making it suitable for 3D culture and organoid construction (Table 3). However, since these materials are derived from animal sources, variability between species is introduced, raising concerns about potential immune rejection. Additionally, further validation is required for their biosafety in clinical translation. Artificially synthesized materials, such as polyethylene glycol (PEG), is not easily biodegradable in the body, which can lead to potential accumulation and complications over time. Additionally, PEG lacks inherent biological activity, meaning it does not naturally promote cell adhesion, tissue regeneration, or other key biological processes necessary for successful integration in tissue engineering applications. DNA-based hydrogels offer several distinct advantages: i. Unlike traditional hydrogels, DNA-based hydrogel has extremely high safety and excellent biomimetic properties. The degradation products of DNA-based hydrogels are deoxynucleotides, which can be fully metabolized by the body without raising biocompatibility concerns. Additionally, as DNA-based hydrogels degrade, they release phosphate ions, which can serve as mineralization agents, further enhancing bone regeneration. This makes DNA-based hydrogels an ideal scaffold material for bone organoid construction. ii. DNA-based hydrogels have excellent programmability and functionality. One of the most significant advantages of DNA-based hydrogels is their programmability and functionality. DNA sequences can be customized to include specific motifs or functional sequences, such as ODNs, tFNA, i-motif, or DNAzymes. This allows DNA-based hydrogels to adapt to the complex and dynamic microenvironment of bone organoids, simulating different stages of bone healing. These characteristics enable precise control over the physical and biochemical environment, which is crucial for directing the growth and differentiation of cells within the organoids. iii. DNA-based hydrogel has excellent printability. DNA-based hydrogels can also be engineered bioinks for 3D bioprinting. Using techniques such as DLP or extrusion printing, these hydrogels can replicate the intricate structure of bone tissue, making them suitable for creating bone organoids that mimic in vivo bone architecture. iv. DNA-based hydrogel has unique scalability. DNA is a widely used tool in molecular biology, and with advances in the field, the synthesis of DNA has become scalable and efficient. The ability to mass-produce functional DNA sequences quickly provides a strong foundation for the future industrialization of bone organoids production. With the inherent advantages of DNA-based hydrogels, positions them as a promising tool for the construction of bone organoids (Fig. 9).
Table 3.
Summary of hydrogel property and applications.
| Hydrogel Type | Advantages | Disadvantages | Applications | Reference |
|---|---|---|---|---|
| DNA-based hydrogels | Programmable Highly customizable Biocompatible Gene delivery |
Complex fabrication Limited mechanical strength |
Biosensing Drug delivery Tissue engineering Gene therapy. |
[98,102,104] |
| GelMA | Excellent biocompatibility Photocrosslinking Easy fabrication Supports cell growth |
Limited mechanical strength Batch-to-batch variation |
Tissue engineering 3D cell culture Regenerative medicine. |
[187] |
| HAMA | Biocompatible Biodegradable Supports cell adhesion Viscoelastic properties |
Batch-to-batch variation Poor stability in vivo |
Cartilage regeneration, Wound healing Drug delivery. |
[188] |
| Alginate | Biocompatible Ionic crosslinking Encapsulating cells |
Batch-to-batch variation Low mechanical strength |
Tissue engineering Cell encapsulation |
[189] |
| PEG | High chemical stability Non-toxic Non-immunogenic Mechanical properties |
Poor cell adhesion Lacks natural bioactive cues |
Drug delivery Tissue engineering. |
[164] |
| Matrigel | Mimics ECM Supports cell growth and differentiation |
Expensive Batch-to-batch variation Limited mechanical strength |
3D cell culture Organoids culture Cancer research Tissue engineering. |
[190] |
| Collagen | Biocompatible Biodegradable Mimics ECM |
Poor mechanical strength Expensive |
Tissue engineering Wound healing Bone regeneration. |
[191] |
| Fibrin | Cell adhesion Migration Biodegradable Thrombin gelation |
Limited mechanical strength Potential immunogenicity |
Wound healing Tissue engineering Drug release |
[192] |
Fig. 9.
Construction and application of bone organoids based on DNA-based hydrogels.
Bone organoids hold immense potential for advancing research in bone-related diseases, offering versatile applications in disease modeling, bone research, and drug screening. These organoids closely replicate the complex microenvironment of human bone tissue, allowing researchers to study the progression and mechanisms of diseases like osteoporosis, osteoarthritis, and bone cancers in vitro. Additionally, bone organoids provide a powerful platform for investigating bone biology, including cellular behavior and bone remodeling, which is crucial for developing new therapeutic strategies. In drug screening, bone organoids enable high-throughput testing of potential treatments with greater predictive accuracy than traditional cell cultures or animal models. This makes them a transformative tool in advancing both basic research and clinical applications, ultimately improving the development of targeted therapies for bone-related diseases.
6. Conclusion and perspective
In this review, we explore the benefits of DNA-based hydrogels, outline their construction methods, and discuss their crucial roles in the development of bone regeneration. These hydrogels possess unique properties such as biomimetics, programmability, versatility, tunable mechanical strength, and the ability to enhance cellular interactions, making them crucial matrices for mimicking the complex architecture of bone tissue. DNA-based hydrogels offer excellent cellular support and protection. Hydrogel microspheres can be constructed using microfluidic technology, while biomimetic bone structures can be created through 3D printing with DNA-based hydrogel precusors. DNA-based hydrogels provide a promising tool for bone regeneration by addressing three critical aspects: osteogenesis, vascularization, and immune modulation. DNA-based hydrogels offer a supportive scaffold that enhances the proliferation and differentiation of osteogenic cells and support angiogenesis by delivering TDNs and facilitating sustained release of growth factors, thereby promoting bone regeneration. Finally, immune regulation is a key factor for the success of bone regeneration. DNA-based hydrogels can modulate immune responses, reduce inflammation, and create optimal conditions for bone regeneration by promoting a balanced immune environment. The advantages of DNA-based hydrogels in bone regeneration are being leveraged as a promising tool for the development of bone organoids.
While DNA-based hydrogels hold significant promise for promoting bone regeneration and constructing bone organoids, their transition from laboratory research to clinical applications will take considerable time. Future studies are likely to focus on overcoming preclinical challenges, including the design, application, and clinical use of DNA-based hydrogels, as well as their commercialization. Addressing these obstacles is essential for advancing the use of DNA-based hydrogels in regenerative medicine and making them a viable option for clinical bone repair and organoid development.
1. Design and Synthesis: DNA-based hydrogels are synthesized using solid-phase synthesis methods, where specific sequences can be edited during synthesis to endow the material with unique functional properties. This allows for the design of highly personalized hydrogels tailored to specific biological mechanisms, key targets, or signaling pathways, providing a foundation for constructing bone organoids at various stages of development. One challenge in creating pure DNA-based hydrogels is their relatively low mechanical strength. However, combining DNA with polymers and nanoparticles can enhance this property. For instance, incorporating magnetic nanoparticles allows DNA-based hydrogels to convert magnetic stimuli into mechanical responses, giving the constructed bone organoids enhanced biomimetic functionality.
2. Hydrogel and Organoid Implantation: Localized percutaneous injection has gained wide recognition for its simplicity and control, making it an attractive method for delivering hydrogels to promote bone repair. This minimally invasive approach is patient-friendly and represents a promising therapeutic option. Currently, the treatment of bone defects often involves surgical procedures, such as autologous bone grafting or allogeneic bone transplantation. However, bone organoids cultured in vitro offer a novel alternative to autologous grafts, reducing the risk of complications associated with bone harvesting from the patient. This approach represents a cutting-edge stem cell therapy strategy, offering new possibilities for regenerative medicine and bone tissue engineering.
3. Large-Scale Production: Currently, DNA synthesis can be conducted in sterile environments, ensuring that both the raw materials and final products meet sterility requirements. However, the high cost of DNA synthesis remains a significant limitation to its broader adoption. Additionally, the construction of DNA-based hydrogel-based bone organoids requires advanced in vitro culture facilities. For these organoids to be successfully translated from laboratory research to clinical applications, there is a need for industry-driven innovation. This would enable the large-scale and standardized production of bone organoids, ensuring their availability for clinical use and overcoming the current challenges of cost and scalability.
4. Application: DNA-based hydrogels possess substantial potential to advance areas such as biosensing, tissue engineering, and clinical translation. Their distinctive properties-programmability, biocompatibility, and ease of functionalization-render them highly adaptable for various applications. In biosensing, DNA-based hydrogels are particularly effective for sensitive disease detection and environmental monitoring. For instance, they can be engineered to detect specific biomarkers in the early stages of diseases, with promising applications in early cancer detection and pathogen identification. Additionally, DNA-based hydrogels can be designed to respond to environmental pollutants, such as heavy metals and toxins, facilitating the development of portable, real-time sensors for environmental monitoring. In tissue engineering, DNA-based hydrogels serve as efficient scaffolds for cell growth and tissue regeneration, paving the way for advancements in regenerative medicine. In clinical translation, DNA-based hydrogels show promise in drug delivery, particularly as targeted carriers for bone-related diseases such as osteoporosis and bone tumors. Future research will focus on developing bone organoid models using the self-assembly properties of DNA-based hydrogels with an emphasis on simulating bone tissue. These models will support drug screening, disease modeling, and provide a platform for personalized medicine.
CRediT authorship contribution statement
Xiang Wu: Writing – original draft, Visualization, Resources. Yan Hu: Writing – original draft, Visualization, Resources. Shihao Sheng: Writing – original draft, Visualization, Resources. Huijian Yang: Writing – original draft, Visualization, Resources. Zuhao Li: Writing – review & editing, Supervision, Conceptualization. Qinglin Han: Writing – review & editing, Supervision, Conceptualization. Qin Zhang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Jiacan Su: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Foundation of Shanghai (22ZR1424900), the National Natural Science Foundation of China (82230071, 82172098, 32471420, 82302397), Fujian Provincial Natural Science Foundation of China (2024J01224) and the Shanghai Committee of Science and Technology Laboratory Animal Research Project (23141900600).
Contributor Information
Zuhao Li, Email: lizuhao1992@163.com.
Qinglin Han, Email: 1975hanql@163.com.
Qin Zhang, Email: sabrina_1985@shu.edu.cn.
Jiacan Su, Email: drsujiacan@163.com.
Data availability
No data was used for the research described in the article.
References
- 1.Lu X.F., Yu S.L., Chen G.J., Zheng W.H., Peng J.F., Huang X.F., Chen L.L. Insight into the roles of melatonin in bone tissue and bone-related diseases. Int. J. Mol. Med. 2021;47(5):19. doi: 10.3892/ijmm.2021.4915. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X.D., Koo S., Kim J.H., Huang X.G., Kong N., Zhang L.Q., Zhou J., Xue J.J., Harris M.B., Tao W., Kim J.S. Nanoscale materials-based platforms for the treatment of bone-related diseases. Matter. 2021;4(9):2727–2764. [Google Scholar]
- 3.Alt V., Thormann U., Ray S., Zahner D., Dürselen L., Lips K., El Khassawna T., Heiss C., Riedrich A., Schlewitz G., Ignatius A., Kampschulte M., von Dewitz H., Heinemann S., Schnettler R., Langheinrich A. A new metaphyseal bone defect model in osteoporotic rats to study biomaterials for the enhancement of bone healing in osteoporotic fractures. Acta Biomater. 2013;9(6):7035–7042. doi: 10.1016/j.actbio.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Gao F., Ma X.W., Wang F.K., Zhou F., Ye J., Yang D.L., Li M., Wang P.F. Injectable multifunctional DNA hydrogel for accelerated wound healing. Chem. Eng. J. 2023;470 [Google Scholar]
- 5.Chen P., Yu C.H., Chen J., Xu L.J., Liu H.J. DNA-based supramolecular hydrogels: from construction strategies to biomedical applications. Chin. Chem. Lett. 2023;34(12) [Google Scholar]
- 6.Wang D.Y., Duan J., Liu J.W., Yi H., Zhang Z.Z., Song H.W., Li Y.C., Zhang K.X. Stimuli-responsive self-degradable DNA hydrogels: design, synthesis, and applications. Adv. Healthcare Mater. 2023;12(16) doi: 10.1002/adhm.202203031. [DOI] [PubMed] [Google Scholar]
- 7.Yan C.Y., Hua Y.L., Guo J.R., Miao P. Programmable DNA hydrogels construction with functional regulations for biosensing applications. Trac. Trends Anal. Chem. 2024;173:12. [Google Scholar]
- 8.Idili A., Vallée-Bélisle A., Ricci F. Programmable pH-triggered DNA nanoswitches. J. Am. Chem. Soc. 2014;136(16):5836–5839. doi: 10.1021/ja500619w. [DOI] [PubMed] [Google Scholar]
- 9.Thang N.H., Chien T.B., Cuong D.X. Polymer-based hydrogels applied in drug delivery: an overview. Gels. 2023;9(7):523. doi: 10.3390/gels9070523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu M., Tang X.H., Li Z.H., Wang W.D., Wang S.P., Li M., Yu Q.L.Y., Xie S.J., Zuo X.L., Chen C. High-throughput DNA synthesis for data storage. Chem. Soc. Rev. 2024;53(9):4463–4489. doi: 10.1039/d3cs00469d. [DOI] [PubMed] [Google Scholar]
- 11.Kim T., Park S., Lee M., Baek S., Lee J.B., Park N. DNA hydrogel microspheres and their potential applications for protein delivery and live cell monitoring. Biomicrofluidics. 2016;10(3) doi: 10.1063/1.4953046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y.Z., Duan X.C., Huang J.Y. DNA hydrogels as functional materials and their biomedical applications. Adv. Funct. Mater. 2024;34(3) [Google Scholar]
- 13.Sun J.F., Gao Y., Yao Y.X., Li Y., Feng M.G., Bai L., Chen X.Y., Ge Y.C., Lin Y.F., Cai X.X. Bone tissue engineering based on sustained release of MiR29c-modified framework nucleic acids from an injectable hydrogel. Chem. Eng. J. 2024;487 [Google Scholar]
- 14.Liu X.Z., Hu J., Ning Y., Xu H.J., Cai H.T., Yang A.F., Shi Z.S., Li Z.H. Aptamer technology and its applications in bone diseases. Cell Transplant. 2023;32:11. doi: 10.1177/09636897221144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H.X., Li M., Lee C.M., Chakraborty S., Kim H.W., Bao G., Leong K.W. CRISPR/Cas9-Based genome editing for disease modeling and therapy: challenges and opportunities for Nonviral delivery. Chem. Rev. 2017;117(15):9874–9906. doi: 10.1021/acs.chemrev.6b00799. [DOI] [PubMed] [Google Scholar]
- 16.Song J.H., Gu J.T., Dang G.P., Li Z.T., Lei C., Li L., Mu Z., Tay F.R., Jiao K., Niu L.N. The immunomodulatory effects of DNA-conjugated collagen scaffolds on bone healing. Chem. Eng. J. 2023;474(15) [Google Scholar]
- 17.Medina S.H., Li S., Howard O.M.Z., Dunlap M., Trivett A., Schneider J.P., Oppenheim J.J. Enhanced immunostimulatory effects of DNA-encapsulated peptide hydrogels. Biomaterials. 2015;53:545–553. doi: 10.1016/j.biomaterials.2015.02.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S.S., Chen X., Geng Z., Su J.C. The horizon of bone organoid: a perspective on construction and application. Bioact. Mater. 2022;18:15–25. doi: 10.1016/j.bioactmat.2022.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J., Zhang L.Q., Lu A.P., Liang C. Organoids as innovative models for bone and joint diseases. Cells. 2023;12(12):1590. doi: 10.3390/cells12121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Y., Cheong E., Kwak J.G., Carpenter R., Shim J.H., Lee J. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci. Adv. 2021;7(4) doi: 10.1126/sciadv.abd6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao Y., Jia H.Y., Cao T.Y., Liu D.S. Supramolecular hydrogels based on DNA self-assembly. Acc. Chem. Res. 2017;50(4):659–668. doi: 10.1021/acs.accounts.6b00524. [DOI] [PubMed] [Google Scholar]
- 22.Li C., Faulkner-Jones A., Dun A.R., Jin J., Chen P., Xing Y.Z., Yang Z.Q., Li Z.B., Shu W.M., Liu D.S., Duncan R.R. Rapid Formation of a supramolecular polypeptide-DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem.-Int. Edit. 2015;54(13):3957–3961. doi: 10.1002/anie.201411383. [DOI] [PubMed] [Google Scholar]
- 23.Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 24.Lancaster M.A., Huch M. Disease modelling in human organoids. Dis. Model. Mech. 2019;12(7):14. doi: 10.1242/dmm.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge L., Wang W.X., Sun X.M., Hou T., Li F. Versatile and programmable DNA logic gates on universal and label-free homogeneous electrochemical platform. Anal. Chem. 2016;88(19):9691–9698. doi: 10.1021/acs.analchem.6b02584. [DOI] [PubMed] [Google Scholar]
- 26.Wang M.X., Li X.X., He F., Li J., Wang H.H., Nie Z. Advances in designer DNA nanorobots enabling programmable functions. Chembiochem. 2022;23(18) doi: 10.1002/cbic.202200119. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande S.R., Hammink R., Nelissen F.H.T., Rowan A.E., Heus H.A. Biomimetic stress sensitive hydrogel controlled by DNA nanoswitches. Biomacromolecules. 2017;18(10):3310–3317. doi: 10.1021/acs.biomac.7b00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morya V., Walia S., Mandal B.B., Ghoroi C., Bhatia D. Functional DNA based hydrogels: development, properties and biological applications. ACS Biomater. Sci. Eng. 2020;6(11):6021–6035. doi: 10.1021/acsbiomaterials.0c01125. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y.Z., Tu J., Wang D.Q., Zhu H.T., Maity S.K., Qu X.M., Bogaert B., Pei H., Zhang H.B. Programmable and multifunctional DNA-based materials for biomedical applications. Adv. Mater. 2018;30(24) doi: 10.1002/adma.201703658. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C., Macfarlane R.J., Young K.L., Choi C.H.J., Hao L.L., Auyeung E., Liu G.L., Zhou X.Z., Mirkin C.A. A general approach to DNA-programmable atom equivalents. Nat. Mater. 2013;12(8):741–746. doi: 10.1038/nmat3647. [DOI] [PubMed] [Google Scholar]
- 31.Lv H., Xie N.L., Li M.Q., Dong M.K., Sun C.Y., Zhang Q., Zhao L., Li J., Zuo X.L., Chen H.B., Wang F., Fan C.H. DNA-based programmable gate arrays for general-purpose DNA computing. Nature. 2023;622(7982):292. doi: 10.1038/s41586-023-06484-9. [DOI] [PubMed] [Google Scholar]
- 32.Gacanin J., Synatschke C.V., Weil T. Biomedical applications of DNA-based hydrogels. Adv. Funct. Mater. 2020;30(4) [Google Scholar]
- 33.Shahbazi M.A., Bauleth-Ramos T., Santos H.A. DNA hydrogel assemblies: bridging synthesis principles to biomedical applications. Adv. Therapeut. 2018;1(4) [Google Scholar]
- 34.Bhoyar L., Mehar P., Chavali K. An overview of DNA degradation and its implications in forensic caseworks, Egypt. J. Forensic Sci. 2024;14(1):19. [Google Scholar]
- 35.Wu D., Lei J.Q., Zhang Z.K., Huang F.H., Buljan M., Yu G.C. Polymerization in living organisms. Chem. Soc. Rev. 2023;52(9):2911–2945. doi: 10.1039/d2cs00759b. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z.G., Chen R.P., Yang S.P., Li S., Gao Z.X. Design and application of stimuli-responsive DNA hydrogels: a review. Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y.J., Chen R.F., Zhou B.N., Dong Y.C., Liu D.S. Rational design of DNA hydrogels based on molecular dynamics of polymers. Adv. Mater. 2024;36(7):27. doi: 10.1002/adma.202307129. [DOI] [PubMed] [Google Scholar]
- 38.Jeon K., Lee C., Lee J.Y., Kim D. DNA hydrogels with programmable condensation, expansion, and degradation for molecular carriers. ACS Appl. Mater. Interfaces. 2024;16(19):24162–24171. doi: 10.1021/acsami.3c17633. [DOI] [PubMed] [Google Scholar]
- 39.Nasiri M., Bahadorani M., Dellinger K., Aravamudhan S., Vivero-Escoto J.L., Zadegan R. Improving DNA nanostructure stability: a review of the biomedical applications and approaches. Int. J. Biol. Macromol. 2024;260 doi: 10.1016/j.ijbiomac.2024.129495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G., Huang H., Zou L., Zhang X.C., Lin X.Y., Javed M., Zhang X.C., Xu Y.Q., Ai R., Luo Z.S., Li D. Intelligent DNA-based hydrogels: an outstanding programable and biocompatible material for food applications. Trends Food Sci. Technol. 2024;149 [Google Scholar]
- 41.Tang J.P., Wang J., Ou J.H., Cui Z., Yao C., Yang D.Y. A DNA/Poly-(L-lysine) hydrogel with long shelf-time for 3D cell culture. Small Methods. 2024;8(7) doi: 10.1002/smtd.202301236. [DOI] [PubMed] [Google Scholar]
- 42.Huang L., Huang H.B., Zhang Z.M., Li G.K. Three-dimensional DNA hydrogel mediated dual-mode sensing method for quantification of epithelial cell adhesion molecule in biological fluid samples. Anal. Chem. 2024;96(30):12254–12261. doi: 10.1021/acs.analchem.4c01006. [DOI] [PubMed] [Google Scholar]
- 43.Wei Y.H., Wang K.Z., Luo S.H., Li F., Zuo X.L., Fan C.H., Li Q. Programmable DNA hydrogels as artificial extracellular matrix. Small. 2022;18(36) doi: 10.1002/smll.202107640. [DOI] [PubMed] [Google Scholar]
- 44.Yao S.T., Chang Y.Y., Zhai Z.J., Sugiyama H., Endo M., Zhu W.P., Xu Y.F., Yang Y.Y., Qian X.H. DNA-based daisy chain rotaxane nanocomposite hydrogels as dual-programmable dynamic scaffolds for stem cell adhesion. ACS Appl. Mater. Interfaces. 2022;14(18):20739–20748. doi: 10.1021/acsami.2c03265. [DOI] [PubMed] [Google Scholar]
- 45.Kahn J.S., Hu Y.W., Willner I. Stimuli-responsive DNA-based hydrogels: from basic principles to applications. Acc. Chem. Res. 2017;50(4):680–690. doi: 10.1021/acs.accounts.6b00542. [DOI] [PubMed] [Google Scholar]
- 46.Li F., Tang J., Geng J., Luo D., Yang D. Polymeric DNA hydrogel: design, synthesis and applications. Prog. Polym. Sci. 2019;98 [Google Scholar]
- 47.Abune L., Davis B., Wang Y. Aptamer-functionalized hydrogels: an emerging class of biomaterials for protein delivery, cell capture, regenerative medicine, and molecular biosensing. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2021;13(6) doi: 10.1002/wnan.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y.Z., Duan X.C., Huang J.Y. DNA hydrogels as functional materials and their biomedical applications. Adv. Funct. Mater. 2023;34(3) [Google Scholar]
- 49.Arnon Z.A., Piperno S., Redeker D.C., Randall E., Tkachenko A.V., Shpaisman H., Gang O. Acoustically shaped DNA-programmable materials. Nat. Commun. 2024;15(1):6875. doi: 10.1038/s41467-024-51049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothemund P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440(7082):297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 51.Li Y.G., Tseng Y.D., Kwon S.Y., D'Espaux L., Bunch J.S., McEuen P.L., Luo D. Controlled assembly of dendrimer-like DNA. Nat. Mater. 2004;3(1):38–42. doi: 10.1038/nmat1045. [DOI] [PubMed] [Google Scholar]
- 52.He Y., Chen Y., Liu H.P., Ribbe A.E., Mao C.D. Self-assembly of hexagonal DNA two-dimensional (2D) arrays. J. Am. Chem. Soc. 2005;127(35):12202–12203. doi: 10.1021/ja0541938. [DOI] [PubMed] [Google Scholar]
- 53.Zheng J.P., Birktoft J.J., Chen Y., Wang T., Sha R.J., Constantinou P.E., Ginell S.L., Mao C.D., Seeman N.C. From molecular to macroscopic via the rational design of a self-assembled 3D DNA crystal. Nature. 2009;461(7260):74–77. doi: 10.1038/nature08274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garneau J.E., Dupuis M.E., Villion M., Romero D.A., Barrangou R., Boyaval P., Fremaux C., Horvath P., Magadán A.H., Moineau S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468(7320):67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 55.Deltcheva E., Chylinski K., Sharma C.M., Gonzales K., Chao Y.J., Pirzada Z.A., Eckert M.R., Vogel J., Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C., Zhang L., Liu H., Cheng K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J. Contr. Release. 2017;266:17–26. doi: 10.1016/j.jconrel.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cong L., Ran F.A., Cox D., Lin S.L., Barretto R., Habib N., Hsu P.D., Wu X.B., Jiang W.Y., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding F., Huang X.G., Gao X.H., Xie M., Pan G.F., Li Q.F., Song J., Zhu X.Y., Zhang C. A non-cationic nucleic acid nanogel for the delivery of the CRISPR/Cas9 gene editing tool. Nanoscale. 2019;11(37):17211–17215. doi: 10.1039/c9nr05233j. [DOI] [PubMed] [Google Scholar]
- 59.Gao Q.Q., Zhang J., Chen C., Chen M.L., Sun P., Du W., Zhang S.C., Liu Y., Zhang R., Bai M., Fan C.C., Wu J.B., Men T.Y., Jiang X.Y. In situ mannosylated nanotrinity-mediated macrophage remodeling combats Candida albicans infection. ACS Nano. 2020;14(4):3980–3990. doi: 10.1021/acsnano.9b07896. [DOI] [PubMed] [Google Scholar]
- 60.Han Y.F., Cao L.H., Li G.F., Zhou F.J., Bai L., Su J.C. Harnessing nucleic acids nanotechnology for bone/cartilage regeneration. Small. 2023;19(37) doi: 10.1002/smll.202301996. [DOI] [PubMed] [Google Scholar]
- 61.Zhao D., Liu M.T., Li J.J., Xiao D.X., Peng S.L., He Q., Sun Y., Li Q.R., Lin Y.F. Angiogenic aptamer-modified tetrahedral framework nucleic acid promotes angiogenesis in vitro and in vivo. ACS Appl. Mater. Interfaces. 2021;13(25):29439–29449. doi: 10.1021/acsami.1c08565. [DOI] [PubMed] [Google Scholar]
- 62.Tian T.R., Zhang T., Shi S.R., Gao Y., Cai X.X., Lin Y.F. A dynamic DNA tetrahedron framework for active targeting. Nat. Protoc. 2023;18(4):1028–1055. doi: 10.1038/s41596-022-00791-7. [DOI] [PubMed] [Google Scholar]
- 63.Walsh A.S., Yin H.F., Erben C.M., Wood M.J.A., Turberfield A.J. DNA cage delivery to mammalian cells. ACS Nano. 2011;5(7):5427–5432. doi: 10.1021/nn2005574. [DOI] [PubMed] [Google Scholar]
- 64.Shi S.R., Peng Q., Shao X.R., Xie J., Lin S.Y., Zhang T., Li Q.S., Li X.L., Lin Y.F. Self-assembled tetrahedral DNA nanostructures promote adipose-derived stem cell migration via IncRNA XLOC 010623 and RHOA/ROCK2 signal pathway. ACS Appl. Mater. Interfaces. 2016;8(30):19353–19363. doi: 10.1021/acsami.6b06528. [DOI] [PubMed] [Google Scholar]
- 65.Xie X., Shao X., Ma W., Zhao D., Shi S., Li Q., Lin Y. Overcoming drug-resistant lung cancer by paclitaxel loaded tetrahedral DNA nanostructures. Nanoscale. 2018;10(12):5457–5465. doi: 10.1039/c7nr09692e. [DOI] [PubMed] [Google Scholar]
- 66.Wang C., Zhang J.J. Recent advances in stimuli-responsive DNA-based hydrogels. ACS Appl. Bio Mater. 2022;5(5):1934–1953. doi: 10.1021/acsabm.1c01197. [DOI] [PubMed] [Google Scholar]
- 67.Berman H.M., Olson W.K., Beveridge D.L., Westbrook J., Gelbin A., Demeny T., Hsieh S.H., Srinivasan A.R., Schneider B. The nucleic acid database. A comprehensive relational database of three-dimensional structures of nucleic acids. Biophys. J. 1992;63(3):751–759. doi: 10.1016/S0006-3495(92)81649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soto A.M., Loo J., Marky L.A. Energetic contributions for the formation of TAT/TAT, TAT/CGC+, and CGC+/CGC+ base triplet stacks. J. Am. Chem. Soc. 2002;124(48):14355–14363. doi: 10.1021/ja026952h. [DOI] [PubMed] [Google Scholar]
- 69.Leroy J.L., Gueron M., Mergny J.L., Helene C. Intramolecular folding of a fragment of the cytosine-rich strand of telomeric DNA into an i-motif. Nucleic Acids Res. 1994;22(9):1600–1606. doi: 10.1093/nar/22.9.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi J., Kim S., Tachikawa T., Fujitsuka M., Majima T. pH-induced intramolecular folding dynamics of i-motif DNA. J. Am. Chem. Soc. 2011;133(40):16146–16153. doi: 10.1021/ja2061984. [DOI] [PubMed] [Google Scholar]
- 71.Min Z.Q., Xu B.Y., Li W., Zhang A. Combination of DNA with polymers. Polym. Chem. 2021;12(13):1898–1917. [Google Scholar]
- 72.Hu Y.W., Ying J.Y. A strong acid-induced DNA hydrogel based on pH-reconfigurable A-motif duplex. Small. 2023;19(12) doi: 10.1002/smll.202205909. [DOI] [PubMed] [Google Scholar]
- 73.Dong Y.C., Yang Z.Q., Liu D.S. DNA nanotechnology based on i-motif structures. Acc. Chem. Res. 2014;47(6):1853–1860. doi: 10.1021/ar500073a. [DOI] [PubMed] [Google Scholar]
- 74.Hu Y.W., Gao S.J., Lu H.F., Ying J.Y. Acid-resistant and physiological pH-responsive DNA hydrogel composed of A-motif and i-motif toward oral insulin delivery. J. Am. Chem. Soc. 2022;144(12):5461–5470. doi: 10.1021/jacs.1c13426. [DOI] [PubMed] [Google Scholar]
- 75.Zeuner R.A., Verthelyi D., Gursel M., Ishii K.J., Klinman D.M. Influence of stimulatory and suppressive DNA motifs on host susceptibility to inflammatory arthritis. Arthritis Rheum. 2003;48(6):1701–1707. doi: 10.1002/art.11035. [DOI] [PubMed] [Google Scholar]
- 76.Shirota H., Gursel I., Gursel M., Klinman D.M. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J. Immunol. 2005;174(8):4579–4583. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 77.Cheng X., Chen Y., Xie J.J., Yao R., Yu X., Liao M.Y., Ding Y.J., Tang T.T., Liao Y.H., Cheng Y. Suppressive oligodeoxynucleotides inhibit atherosclerosis in ApoE-/-mice through modulation of Th1/Th2 balance. J. Mol. Cell. Cardiol. 2008;45(2):168–175. doi: 10.1016/j.yjmcc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 78.Sato T., Shimosato T., Alvord W.G., Klinman D.M. Suppressive oligodeoxynucleotides inhibit silica-induced pulmonary inflammation. J. Immunol. 2008;180(11):7648–7654. doi: 10.4049/jimmunol.180.11.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong L., Ito S.I., Ishii K.J., Klinman D.M. Suppressive oligonucleotides protect against collagen-induced arthritis in mice. Arthritis Rheum. 2004;50(5):1686–1689. doi: 10.1002/art.20263. [DOI] [PubMed] [Google Scholar]
- 80.Yamada H., Gursel I., Takeshita F., Conover J., Ishii K.J., Gursel M., Takeshita S., Klinman D.M. Effect of suppressive DNA on CpG-induced immune activation. J. Immunol. 2002;169(10):5590–5594. doi: 10.4049/jimmunol.169.10.5590. [DOI] [PubMed] [Google Scholar]
- 81.Li W.S., Luo L., Huang J., Wang Q., Liu J.B., Xiao X., Fang H.M., Yang X.H., Wang K.M. Self-assembled DNA nanocentipedes as multivalent vehicles for enhanced delivery of CpG oligonucleotides. Chem. Commun. 2017;53(40):5565–5568. doi: 10.1039/c7cc01128h. [DOI] [PubMed] [Google Scholar]
- 82.Odeh F., Nsairat H., Alshaer W., Ismail M.A., Esawi E., Qaqish B., Al Bawab A., Ismail S.I. Aptamers chemistry: chemical modifications and conjugation strategies. Molecules. 2020;25(1) doi: 10.3390/molecules25010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ni S.J., Zhuo Z.J., Pan Y.F., Yu Y.Y., Li F.F., Liu J., Wang L.Y., Wu X.Q., Li D.J., Wan Y.Y., Zhang L.H., Yang Z.J., Zhang B.T., Lu A.P., Zhang G. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl. Mater. Interfaces. 2021;13(8):9500–9519. doi: 10.1021/acsami.0c05750. [DOI] [PubMed] [Google Scholar]
- 84.Byun J. Recent progress and opportunities for nucleic acid aptamers. Life-Basel. 2021;11(3):193. doi: 10.3390/life11030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muhammad M., Huang Q. A review of aptamer-based SERS biosensors: design strategies and applications. Talanta. 2021;227(15) doi: 10.1016/j.talanta.2021.122188. [DOI] [PubMed] [Google Scholar]
- 86.Zhou J.H., Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 2017;16(3):181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayashi T., Oshima H., Mashima T., Nagata T., Katahira M., Kinoshita M. Binding of an RNA aptamer and a partial peptide of a prion protein: crucial importance of water entropy in molecular recognition. Nucleic Acids Res. 2014;42(11):6861–6875. doi: 10.1093/nar/gku382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rana D., Padmanaban P., Becker M., Stein F., Leijten J., Koopman B., Rouwkema J. Spatial control of self-organizing vascular networks with programmable aptamer-tethered growth factor photopatterning. Mater. Today Bio. 2023;19 doi: 10.1016/j.mtbio.2023.100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richardson S.M., Wheelan S.J., Yarrington R.M., Boeke J.D. GeneDesign: rapid, automated design of multikilobase synthetic genes. Genome Res. 2006;16(4):550–556. doi: 10.1101/gr.4431306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nihashi Y., Miyoshi M., Umezawa K., Shimosato T., Takaya T. Identification of a novel osteogenetic oligodeoxynucleotide (osteoDN) that promotes osteoblast differentiation in a TLR9-independent manner. Nanomaterials. 2022;12(10):1680. doi: 10.3390/nano12101680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seeman N.C., Sleiman H.F. DNA nanotechnology. Nat. Rev. Mater. 2018;3(1) [Google Scholar]
- 92.Um S.H., Lee J.B., Park N., Kwon S.Y., Umbach C.C., Luo D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006;5(10):797–801. doi: 10.1038/nmat1741. [DOI] [PubMed] [Google Scholar]
- 93.Xing Y.Z., Cheng E.J., Yang Y., Chen P., Zhang T., Sun Y.W., Yang Z.Q., Liu D.S. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 2011;23(9):1117–1121. doi: 10.1002/adma.201003343. [DOI] [PubMed] [Google Scholar]
- 94.Guo W.W., Lu C.H., Orbach R., Wang F.A., Qi X.J., Cecconello A., Seliktar D., Willner I. pH-stimulated DNA hydrogels exhibiting shape-memory properties. Adv. Mater. 2015;27(1):73–78. doi: 10.1002/adma.201403702. [DOI] [PubMed] [Google Scholar]
- 95.Iqbal S., Ahmed F., Xiong H. Responsive-DNA hydrogel based intelligent materials: preparation and applications. Chem. Eng. J. 2021;420 [Google Scholar]
- 96.Singh S., Mishra A., Kumari R., Sinha K.K., Singh M.K., Das P. Carbon dots assisted formation of DNA hydrogel for sustained release of drug. Carbon. 2017;114:169–176. [Google Scholar]
- 97.Tang J.P., Yao C., Gu Z., Jung S.W., Luo D., Yang D.Y. Super-Soft and super-elastic DNA robot with magnetically driven navigational locomotion for cell delivery in confined space. Angew. Chem.-Int. Edit. 2020;59(6):2490–2495. doi: 10.1002/anie.201913549. [DOI] [PubMed] [Google Scholar]
- 98.Hu Y., Dominguez C.M., Bauer J., Weigel S., Schipperges A., Oelschlaeger C., Willenbacher N., Keppler S., Bastmeyer M., Heissler S., Wöll C., Scharnweber T., Rabe K.S., Niemeyer C.M. Carbon-nanotube reinforcement of DNA-silica nanocomposites yields programmable and cell-instructive biocoatings. Nat. Commun. 2019;10:5522. doi: 10.1038/s41467-019-13381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orr A., Wilson P., Stotesbury T. DNA-crosslinked alginate hydrogels: characterization, microparticle development, and applications in forensic science. ACS Appl. Polym. Mater. 2023;5(1):583–592. [Google Scholar]
- 100.Barker K., Rastogi S.K., Dominguez J., Cantu T., Brittain W., Irvin J., Betancourt T. Biodegradable DNA-enabled poly(ethylene glycol) hydrogels prepared by copper-free click chemistry. J. Biomater. Sci. Polym. Ed. 2016;27(1):22–39. doi: 10.1080/09205063.2015.1103590. [DOI] [PubMed] [Google Scholar]
- 101.Grajkowski A., Cieslak J., Beaucage S.L. Solid-phase purification of synthetic DNA sequences. J. Org. Chem. 2016;81(15):6165–6175. doi: 10.1021/acs.joc.6b01020. [DOI] [PubMed] [Google Scholar]
- 102.Lee J., Peng S.M., Yang D.Y., Roh Y.H., Funabashi H., Park N., Rice E.J., Chen L.W., Long R., Wu M.M., Luo D. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012;7(12):816–820. doi: 10.1038/nnano.2012.211. [DOI] [PubMed] [Google Scholar]
- 103.Li F., Lv Z.Y., Zhang X., Dong Y.H., Ding X.H., Li Z.M., Li S., Yao C., Yang D.Y. Supramolecular self-assembled DNA nanosystem for synergistic chemical and gene regulations on cancer cells. Angew. Chem.-Int. Edit. 2021;60(48):25557–25566. doi: 10.1002/anie.202111900. [DOI] [PubMed] [Google Scholar]
- 104.Wang J.B., Chao J., Liu H.J., Su S., Wang L.H., Huang W., Willner I., Fan C.H. Clamped hybridization chain reactions for the self-assembly of patterned DNA hydrogels. Angew. Chem.-Int. Edit. 2017;56(8):2171–2175. doi: 10.1002/anie.201610125. [DOI] [PubMed] [Google Scholar]
- 105.Guo W.W., Qi X.J., Orbach R., Lu C.H., Freage L., Mironi-Harpaz I., Seliktar D., Yang H.H., Willner I. Reversible Ag+ crosslinked DNA hydrogels. Chem. Commun. 2014;50(31):4065–4068. doi: 10.1039/c3cc49140d. [DOI] [PubMed] [Google Scholar]
- 106.Geng J.H., Yao C., Kou X.H., Tang J.P., Luo D., Yang D.Y. A fluorescent biofunctional DNA hydrogel prepared by enzymatic polymerization. Adv. Healthcare Mater. 2018;7(5) doi: 10.1002/adhm.201700998. [DOI] [PubMed] [Google Scholar]
- 107.Sun L.P., Hu N., Peng J., Chen L.Y., Weng J. Ultrasensitive detection of mitochondrial DNA mutation by graphene oxide/DNA hydrogel electrode. Adv. Funct. Mater. 2014;24(44):6905–6913. [Google Scholar]
- 108.Cheng E.J., Li Y.L., Yang Z.Q., Deng Z.X., Liu D.S. DNA-SWNT hybrid hydrogel. Chem. Commun. 2011;47(19):5545–5547. doi: 10.1039/c1cc11028d. [DOI] [PubMed] [Google Scholar]
- 109.Ma X.Z., Yang Z.Q., Wang Y.J., Zhang G.L., Shao Y., Jia H.Y., Cao T.Y., Wang R., Liu D.S. Remote controlling DNA hydrogel by magnetic field. ACS Appl. Mater. Interfaces. 2017;9(3):1995–2000. doi: 10.1021/acsami.6b12327. [DOI] [PubMed] [Google Scholar]
- 110.Yang D.Y., Peng S.M., Hartman M.R., Gupton-Campolongo T., Rice E.J., Chang A.K., Gu Z., Lu G.Q., Luo D. Enhanced transcription and translation in clay hydrogel and implications for early life evolution. Sci. Rep. 2013;3:3165. doi: 10.1038/srep03165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu Y.W., Lu C.H., Guo W.W., Aleman-Garcia M.A., Ren J.T., Willner I. A shape memory acrylamide/DNA hydrogel exhibiting switchable dual pH-responsiveness. Adv. Funct. Mater. 2015;25(44):6867–6874. [Google Scholar]
- 112.Alafeef M., Srivastava I., Aditya T., Pan D. Carbon dots: from synthesis to unraveling the fluorescence mechanism. Small. 2024;20(4) doi: 10.1002/smll.202303937. [DOI] [PubMed] [Google Scholar]
- 113.Josephson T.O., Morgan E.F. Harnessing mechanical cues in the cellular microenvironment for bone regeneration. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1232698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gu Q.G., Tian H.J., Zhang K., Chen D.Y., Chen D.C., Wang X.W., Zhao J. Wnt5a/FZD4 mediates the mechanical stretch-induced osteogenic differentiation of bone mesenchymal stem cells, cell. Physiol. Biochem. 2018;48(1):215–226. doi: 10.1159/000491721. [DOI] [PubMed] [Google Scholar]
- 115.Yamada S., Yassin M.A., Torelli F., Hansmann J., Green J.B.A., Schwarz T., Mustafa K. Unique osteogenic profile of bone marrow stem cells stimulated in perfusion bioreactor is Rho-ROCK-mediated contractility dependent. Bioeng. Transl. Med. 2023;8(3) doi: 10.1002/btm2.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J., Zhou D.Y., Wang G.C., Wang M.M., Wang F.X., Han Y.F., Chen X., Hu Y., Zhou F.J., Shi Z.M., Ren X.X., Su J.C. Enhanced bone regeneration with bioprinted GelMA/Bentonite scaffolds inspired by bone matrix. Virtual Phys. Prototyp. 2024;19(1) [Google Scholar]
- 117.Desai N., Pande S., Vora L.K., Kommineni N. Nanofibrous microspheres: a biomimetic platform for bone tissue regeneration. ACS Appl. Bio Mater. 2024;7(9):6325–6331. doi: 10.1021/acsabm.4c01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Budharaju H., Sundaramurthi D., Sethuraman S. Embedded 3D bioprinting - an emerging strategy to fabricate biomimetic & large vascularized tissue constructs. Bioact. Mater. 2024;32:356–384. doi: 10.1016/j.bioactmat.2023.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan X., Yang B., Chen Y.R., Song Y.F., Ye J., Pan Y.F., Zhou B.N., Wang Y.Q., Mao F.B.A., Dong Y.C., Liu D.S., Yu J.K. Anti-friction MSCs delivery system improves the therapy for severe osteoarthritis. Adv. Mater. 2021;33(52) doi: 10.1002/adma.202104758. [DOI] [PubMed] [Google Scholar]
- 120.Foster A.A., Marquardt L.M., Heilshorn S.C. The diverse roles of hydrogel mechanics in injectable stem cell transplantation. Curr. Opin. Chem. Eng. 2017;15:15–23. doi: 10.1016/j.coche.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xie C., Liang R.J., Ye J.C., Peng Z., Sun H., Zhu Q.W., Shen X.L., Hong Y., Wu H.W., Sun W., Yao X.D., Li J.J., Zhang S.F., Zhang X.Z., Ouyang H.W. High-efficient engineering of osteo-callus organoids for rapid bone regeneration within one month. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121741. [DOI] [PubMed] [Google Scholar]
- 122.Yang Z., Wang B., Liu W., Li X., Liang K., Fan Z., Li J.J., Niu Y., He Z., Li H., Wang D., Lin J., Du Y., Lin J., Xing D. In situ self-assembled organoid for osteochondral tissue regeneration with dual functional units. Bioact. Mater. 2023;27:200–215. doi: 10.1016/j.bioactmat.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lück S., Schubel R., Rüb J., Hahn D., Mathieu E., Zimmermann H., Scharnweber D., Werner C., Pautot S., Jordan R. Tailored and biodegradable poly(2-oxazoline) microbeads as 3D matrices for stem cell culture in regenerative therapies. Biomaterials. 2016;79:1–14. doi: 10.1016/j.biomaterials.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 124.Yu C., Kornmuller A., Brown C., Hoare T., Flynn L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials. 2017;120:66–80. doi: 10.1016/j.biomaterials.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 125.Daley E.L.H., Coleman R.M., Stegemann J.P. Biomimetic microbeads containing a chondroitin sulfate/chitosan polyelectrolyte complex for cell-based cartilage therapy. J. Mater. Chem. B. 2015;3(40):7920–7929. doi: 10.1039/C5TB00934K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y.S., Yuan X.L., Yu K., Meng H.Y., Zheng Y.D., Peng J., Lu S.B., Liu X.T., Xie Y.J., Qiao K. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials. 2018;171:118–132. doi: 10.1016/j.biomaterials.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 127.Shen C., Wang J., Li G., Hao S., Wu Y., Song P., Han Y., Li M., Wang G., Xu K., Zhang H., Ren X., Jing Y., Yang R., Geng Z., Su J. Boosting cartilage repair with silk fibroin-DNA hydrogel-based cartilage organoid precursor. Bioact. Mater. 2024;35:429–444. doi: 10.1016/j.bioactmat.2024.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J., Wu Y., Li G.F., Zhou F.J., Wu X., Wang M.M., Liu X.R., Tang H., Bai L., Geng Z., Song P.R., Shi Z.M., Ren X.X., Su J.C. Engineering large-scale self-mineralizing bone organoids with bone matrix-inspired hydroxyapatite hybrid bioinks. Adv. Mater. 2024 doi: 10.1002/adma.202309875. [DOI] [PubMed] [Google Scholar]
- 129.Yu X.G., Gholipourmalekabadi M., Wang X.D., Yuan C.Y., Lin K.L. Three-dimensional bioprinting biphasic multicellular living scaffold facilitates osteochondral defect regeneration. Interdisciplinary Materials. 2024;3(5):738–756. [Google Scholar]
- 130.Lewns F.K., Tsigkou O., Cox L.R., Wildman R.D., Grover L.M., Poologasundarampillai G. Hydrogels and bioprinting in bone tissue engineering: creating artificial stem-cell niches for in vitro models. Adv. Mater. 2023;35(52):20. doi: 10.1002/adma.202301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu Y.R., Yu X.G., Liu H., Li J.J., Gholipourmalekabadi M., Lin K.L., Yuan C.Y., Wang P.L. Strategies of functionalized GelMA-based bioinks for bone regeneration: recent advances and future perspectives. Bioact. Mater. 2024;38:346–373. doi: 10.1016/j.bioactmat.2024.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y., Li G.F., Wang J., Zhou F.J., Ren X.X., Su J.C. Small joint organoids 3D bioprinting: construction strategy and application. Small. 2024;20(8) doi: 10.1002/smll.202302506. [DOI] [PubMed] [Google Scholar]
- 133.Tavakoli S., Krishnan N., Mokhtari H., Oommen O.P., Varghese O.P. Fine-tuning dynamic cross-linking for enhanced 3D bioprinting of hyaluronic acid hydrogels. Adv. Funct. Mater. 2024;34(4) [Google Scholar]
- 134.Li H.B., Dai J.L., Wang Z.X., Zheng H.S., Li W.L., Wang M.A., Cheng F. Digital light processing (DLP)-based (bio)printing strategies for tissue modeling and regeneration. Aggregate. 2023;4(2):e270. [Google Scholar]
- 135.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 136.Chen W.K., Zhang H., Zhou Q.R., Zhou F.J., Zhang Q., Su J.C. Smart hydrogels for bone reconstruction via modulating the microenvironment. Research. 2023;6:89. doi: 10.34133/research.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zare I., Taheri-Ledari R., Esmailzadeh F., Salehi M.M., Mohammadi A., Maleki A., Mostafavi E. DNA hydrogels and nanogels for diagnostics, therapeutics, and theragnostics of various cancers. Nanoscale. 2023;15(26):10882–10903. doi: 10.1039/d3nr00425b. [DOI] [PubMed] [Google Scholar]
- 138.Zhou B.N., Li C.F., Liu D.S., Liu W.L. Chemical strategies and biomedical applications of DNA hydrogels. Fundamental Res. 2023;3(4):534–536. doi: 10.1016/j.fmre.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu X.G., Jing X.X., Liu P., Pan M.C., Liu Z., Dai X.P., Lin J.P., Li Q., Wang F., Yang S.C., Wang L.H., Fan C.H. DNA framework-encoded mineralization of calcium phosphate. Chem. 2020;6(2):472–485. [Google Scholar]
- 140.Athanasiadou D., Carneiro K.M.M. DNA nanostructures as templates for biomineralization. Nat. Rev. Chem. 2021;5(2):93–108. doi: 10.1038/s41570-020-00242-5. [DOI] [PubMed] [Google Scholar]
- 141.Danesi A.L., Athanasiadou D., Aderinto A.O., Rasie P., Chou L.Y.T., Carneiro K.M.M. Peptide-decorated DNA nanostructures promote site-specific hydroxyapatite growth. ACS Appl. Mater. Interfaces. 2022;14(1):1692–1698. doi: 10.1021/acsami.1c19271. [DOI] [PubMed] [Google Scholar]
- 142.Kim F., Chen T., Burgess T., Rasie P., Selinger T.L., Greschner A., Rizis G., Carneiro K. Functionalized DNA nanostructures as scaffolds for guided mineralization. Chem. Sci. 2019;10(45):10537–10542. doi: 10.1039/c9sc02811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Athanasiadou D., Meshry N., Monteiro N.G., Ervolino-Silva A.C., Chan R.L., McCulloch C.A., Okamoto R., Carneiro K.M.M. DNA hydrogels for bone regeneration. Proc. Natl. Acad. Sci. U.S.A. 2023;120(17) doi: 10.1073/pnas.2220565120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Katsumata Y., Kajiya H., Okabe K., Fukushima T., Ikebe T. A salmon DNA scaffold promotes osteogenesis through activation of sodium-dependent phosphate cotransporters. Biochem. Biophys. Res. Commun. 2015;468(4):622–628. doi: 10.1016/j.bbrc.2015.10.172. [DOI] [PubMed] [Google Scholar]
- 145.Beck G.R., Zerler B., Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. U.S.A. 2000;97(15):8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chai Y.C., Carlier A., Bolander J., Roberts S.J., Geris L., Schrooten J., Van Oosterwyck H., Luyten F.P. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater. 2012;8(11):3876–3887. doi: 10.1016/j.actbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 147.Wu H.P., Huang S., Zhu Y., Li J., Pang X.X., Tang Y.Y., Li S.Q., Ji P., Ding S.J., Cheng W., Li W.Y. Self-mineralizing dnazyme hydrogel as a multifaceted bone microenvironment amendment for promoting osteogenesis in osteoporosis. Adv. Funct. Mater. 2023;33(19) [Google Scholar]
- 148.Choi H.J., Kim Y.A., Ryu J., Park K.K., Lee S.J., Kim B.S., Song J.E., Kim J.D. STAT3 decoy oligodeoxynucleotides suppress liver inflammation and fibrosis in liver cancer cells and a DDC-induced liver injury mouse model. Molecules. 2024;29(3):593. doi: 10.3390/molecules29030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hou X., Shen Y.Q., Zhang C., Zhang L.R., Qin Y.Y., Yu Y.L., Wang L.Y., Sun X.H. A specific oligodeoxynucleotide promotes the differentiation of osteoblasts via ERK and p38 MAPK pathways. Int. J. Mol. Sci. 2012;13(7):7902–7914. doi: 10.3390/ijms13077902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amcheslavsky A., Hemmi H., Akira S., Bar-Shavit Z. Differential contribution of osteoclast- and osteoblast-lineage cells to CpG-oligodeoxynucleotide (CpG-ODN) modulation of osteoclastogenesis. J. Bone Miner. Res. 2005;20(9):1692–1699. doi: 10.1359/JBMR.050515. [DOI] [PubMed] [Google Scholar]
- 151.Feng Z.Y., Shen Y.Q., Wang L.Y., Cheng L., Wang J., Li Q.S., Shi W., Sun X.H. An oligodeoxynucleotide with promising modulation activity for the proliferation and activation of osteoblast. Int. J. Mol. Sci. 2011;12(4):2543–2555. doi: 10.3390/ijms12042543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shen Y.Q., Feng Z.Y., Lin C.T., Hou X., Wang X.J., Wang J., Yu Y.L., Wang L.Y., Sun X.H. An oligodeoxynucleotide that induces differentiation of bone marrow mesenchymal stem cells to osteoblasts in vitro and reduces alveolar bone loss in rats with periodontitis. Int. J. Mol. Sci. 2012;13(3):2877–2892. doi: 10.3390/ijms13032877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang Q., Qu X.Y., Liang C., Li H.Y., Du S.Y., Wang C., Xie Y.D., Zheng Y., Wang L. Effect of oligonucleotide MT01 delivered by N-isopropylacrylamide modified polyethyleneimine for bone regeneration. Front. Bioeng. Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1204571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee H., Lytton-Jean A.K.R., Chen Y., Love K.T., Park A.I., Karagiannis E.D., Sehgal A., Querbes W., Zurenko C.S., Jayaraman M., Peng C.G., Charisse K., Borodovsky A., Manoharan M., Donahoe J.S., Truelove J., Nahrendorf M., Langer R., Anderson D.G. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 2012;7(6):389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li S.H., Tian T.R., Zhang T., Cai X.X., Lin Y.F. Advances in biological applications of self-assembled DNA tetrahedral nanostructures. Mater. Today. 2019;24:57–68. [Google Scholar]
- 156.Shang R.F., Lee S., Senavirathne G., Lai E.C. microRNAs in action: biogenesis, function and regulation. Nat. Rev. Genet. 2023;24(12):816–833. doi: 10.1038/s41576-023-00611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li S.H., Liu Y.H., Tian T.R., Zhang T., Lin S.Y., Zhou M., Zhang X.L., Lin Y.F., Cai X.X. Bioswitchable delivery of microRNA by framework nucleic acids: application to bone regeneration. Small. 2021;17(47) doi: 10.1002/smll.202104359. [DOI] [PubMed] [Google Scholar]
- 158.Bessa P.C., Casal M., Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J. Tissue Eng. Regen. Med. 2008;2(2–3):81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 159.Khojasteh A., Behnia H., Naghdi N., Esmaeelinejad M., Alikhassy Z., Stevens M. Effects of different growth factors and carriers on bone regeneration: a systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013;116(6):E405–E423. doi: 10.1016/j.oooo.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 160.Hao S.Y., Wang M.K., Yin Z.F., Jing Y.Y., Bai L., Su J.C. Microenvironment-targeted strategy steers advanced bone regeneration. Mater. Today Bio. 2023;22 doi: 10.1016/j.mtbio.2023.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sefkow-Werner J., Machillot P., Sales A., Castro-Ramirez E., Degardin M., Boturyn D., Cavalcanti-Adam E.A., Albiges-Rizo C., Picart C., Migliorini E. Heparan sulfate co-immobilized with cRGD ligands and BMP2 on biomimetic platforms promotes BMP2-mediated osteogenic differentiation. Acta Biomater. 2020;114(15):90–103. doi: 10.1016/j.actbio.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 162.Yang Z.J., Li X.J., Gan X.Q., Wei M.Y., Wang C.B., Yang G.D., Zhao Y.M., Zhu Z.L., Wang Z.S. Hydrogel armed with Bmp2 mRNA-enriched exosomes enhances bone regeneration. J. Nanobiotechnol. 2023;21(1):119. doi: 10.1186/s12951-023-01871-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gong J.S., Zhu G.Q., Zhang Y., Chen B., Liu Y.W., Li H.M., He Z.H., Zou J.T., Qian Y.X., Zhu S., Hu X.Y., Rao S.S., Cao J., Xie H., Wang Z.X., Du W. Aptamer-functionalized hydrogels promote bone healing by selectively recruiting endogenous bone marrow mesenchymal stem cells. Mater. Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang Q., Chen W.K., Li G.F., Ma Z.X., Zhu M.R., Gao Q.M., Xu K., Liu X.R., Lu W.Y., Zhang W.C., Wu Y., Shi Z.M., Su J.C. A factor-free hydrogel with ROS scavenging and responsive degradation for enhanced diabetic bone healing. Small. 2024;20(24) doi: 10.1002/smll.202306389. [DOI] [PubMed] [Google Scholar]
- 165.Carlier A., van Gastel N., Geris L., Carmeliet G., Van Oosterwyck H. Bringing regenerating tissues to life: the importance of angiogenesis in tissue engineering. Angiogenesis. 2014;17(3):735. 735. [Google Scholar]
- 166.Kanczler J.M., Oreffo R.O.C. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cell. Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 167.Chen G., Deng S.H., Zuo M.X., Wang J., Cheng D., Chen B. Non-viral CRISPR activation system targeting VEGF-A and TGF-81 for enhanced osteogenesis of pre-osteoblasts implanted with dual-crosslinked hydrogel. Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang T., Tian T.R., Zhou R.H., Li S.H., Ma W.J., Zhang Y.X., Liu N.X., Shi S.R., Li Q.S., Xie X.P., Ge Y.C., Liu M.T., Zhang Q., Lin S.Y., Cai X.X., Lin Y.F. Design, fabrication and applications of tetrahedral DNA nanostructure-based multifunctional complexes in drug delivery and biomedical treatment. Nat. Protoc. 2020;15(8):2728–2757. doi: 10.1038/s41596-020-0355-z. [DOI] [PubMed] [Google Scholar]
- 169.Han Y., Wu Y., Wang F., Li G., Wang J., Wu X., Deng A., Ren X., Wang X., Gao J., Shi Z., Bai L., Su J. Heterogeneous DNA hydrogel loaded with Apt02 modified tetrahedral framework nucleic acid accelerated critical-size bone defect repair. Bioact. Mater. 2024;35:1–16. doi: 10.1016/j.bioactmat.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Juhl O., Zhao N., Merife A.B., Cohen D., Friedman M., Zhang Y., Schwartz Z., Wang Y., Donahue H. Aptamer-functionalized fibrin hydrogel improves vascular endothelial growth factor release kinetics and enhances angiogenesis and osteogenesis in critically sized cranial defects. ACS Biomater. Sci. Eng. 2019;5(11):6152–6160. doi: 10.1021/acsbiomaterials.9b01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jing X., Wang S., Tang H., Li D.Z., Zhou F.Y., Xin L.J., He Q.Q., Hu S.S., Zhang T.W., Chen T., Song J.L. Dynamically bioresponsive DNA hydrogel incorporated with dual-functional stem cells from apical papilla-derived exosomes promotes diabetic bone regeneration. ACS Appl. Mater. Interfaces. 2022;14(14):16082–16099. doi: 10.1021/acsami.2c02278. [DOI] [PubMed] [Google Scholar]
- 172.Arron J.R., Choi Y. Osteoimmunology - bone versus immune system. Nature. 2000;408(6812):535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 173.Wang Y.L., Zhang H., Hu Y., Jing Y.Y., Geng Z., Su J.C. Bone repair biomaterials: a perspective from immunomodulatory. Adv. Funct. Mater. 2022;32(51) [Google Scholar]
- 174.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012;8(3):133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 175.Xiong Y., Mi B., Lin Z., Hu Y., Yu L., Zha K., Panayi Adriana C., Yu T., Chen L., Liu Z., Patel A., Feng Q., Zhou S., Liu G. Role of the immune microenvironment in bone,cartilage,and soft tissue regeneration:from mechanism to therapeutic opportunity. Militar. Med. Res. 2023;10(4):499–528. doi: 10.1186/s40779-022-00426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xie Y.J., Hu C., Feng Y., Li D.F., Ai T.T., Huang Y.L., Chen X.D., Huang L.J., Tan J.L. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regenerative Biomaterials. 2020;7(3):233–245. doi: 10.1093/rb/rbaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Julier Z., Park A.J., Briquez P.S., Martino M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 178.Zhou M., Liu N.X., Shi S.R., Li Y., Zhang Q., Ma Q.Q., Tian T.R., Ma W.J., Cai X.X., Lin Y.F. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomed. Nanotechnol. Biol. Med. 2018;14(4):1227–1236. doi: 10.1016/j.nano.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 179.Zhou M., Gao S.J.Y., Zhang X.L., Zhang T.X., Zhang T., Tian T.R., Li S.H., Lin Y.F., Cai X.X. The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions. Bioact. Mater. 2021;6(6):1676–1688. doi: 10.1016/j.bioactmat.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guéry L., Dubrot J., Lippens C., Brighouse D., Malinge P., Irla M., Pot C., Reith W., Waldburger J.M., Hugues S. Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression. Cancer Res. 2014;74(22):6430–6440. doi: 10.1158/0008-5472.CAN-14-1149. [DOI] [PubMed] [Google Scholar]
- 181.Li W., Wang C.S., Wang Z.H., Gou L.P., Zhou Y., Peng G., Zhu M., Zhang J.Y., Li R.Q., Ni H.F., Wu L., Zhang W.L., Liu J.Y., Tian Y.L., Chen Z., Han Y.P., Tong N.W., Fu X.H., Zheng X.F., Berggren P.O. Physically cross-linked DNA hydrogel-based sustained cytokine delivery for in situ diabetic alveolar bone rebuilding. ACS Appl. Mater. Interfaces. 2022;14(22):25173–25182. doi: 10.1021/acsami.2c04769. [DOI] [PubMed] [Google Scholar]
- 182.Roy A., Bulut O., Some S., Mandal A.K., Yilmaz M.D. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673–2702. doi: 10.1039/c8ra08982e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang M., Lo E.C.M. Compare the physicochemical and biological properties of engineered polymer-functionalized silver nanoparticles against Porphyromonas gingivalis. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.985708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Peng G., Li W., Peng L.R., Li R.Q., Wang Z.H., Zhou Y., Gou L.P., Zhu X.Y., Xie Q.X., Zhang X.Y., Shen S.M., Wu L., Hu L.Q., Wang C.S., Zheng X.F., Tong N.W. Multifunctional DNA-based hydrogel promotes diabetic alveolar bone defect reconstruction. Small. 2023;20(10) doi: 10.1002/smll.202305594. [DOI] [PubMed] [Google Scholar]
- 185.Chen W.K., Sheng S.H., Tan K., Wang S.C., Wu X., Yang J.Y., Hu Y., Cao L.H., Xu K., Zhou F.J., Su J.C., Zhang Q., Yang L. Injectable hydrogels for bone regeneration with tunable degradability via peptide chirality modification. Mater. Horiz. 2024;11(18):4367–4377. doi: 10.1039/d4mh00398e. [DOI] [PubMed] [Google Scholar]
- 186.Chen W.K., Zhou Z.Y., Chen D.G., Li Y.H., Zhang Q., Su J.C. Bone regeneration using MMP-cleavable peptides-based hydrogels. Gels. 2021;7(4):199. doi: 10.3390/gels7040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang H.J., Zhou Z.Y., Zhang F.J., Wan C. Hydrogel-based 3D bioprinting technology for articular cartilage regenerative engineering. Gels. 2024;10(7):430. doi: 10.3390/gels10070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ren Y.Z., Wang Q., Xu W.L., Yang M.C., Guo W.H., He S.Q., Liu W.T. Alginate-based hydrogels mediated biomedical applications: a review. Int. J. Biol. Macromol. 2024;279 doi: 10.1016/j.ijbiomac.2024.135019. [DOI] [PubMed] [Google Scholar]
- 190.Li X.L., Sheng S.H., Li G.F., Hu Y., Zhou F.J., Geng Z., Su J.C. Research progress in hydrogels for cartilage organoids. Adv. Healthcare Mater. 2024;13(22) doi: 10.1002/adhm.202400431. [DOI] [PubMed] [Google Scholar]
- 191.Liang X., Huang C., Liu H., Chen H., Shou J., Cheng H., Liu G. Natural hydrogel dressings in wound care: design, advances, and perspectives. Chin. Chem. Lett. 2024;35(10) [Google Scholar]
- 192.Li S.J., Dan X., Chen H., Li T., Liu B., Ju Y.K., Li Y., Lei L.J., Fan X. Developing fibrin-based biomaterials/scaffolds in tissue engineering. Bioact. Mater. 2024;40:597–623. doi: 10.1016/j.bioactmat.2024.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.