Abstract
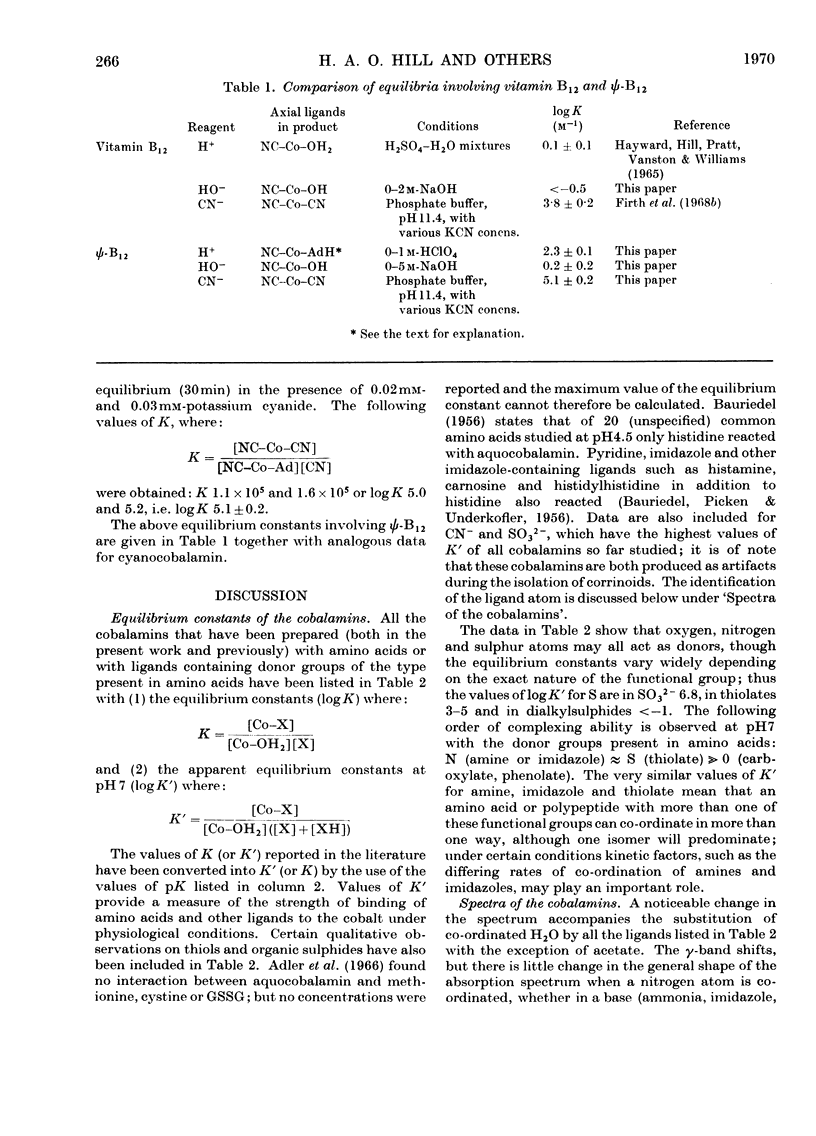
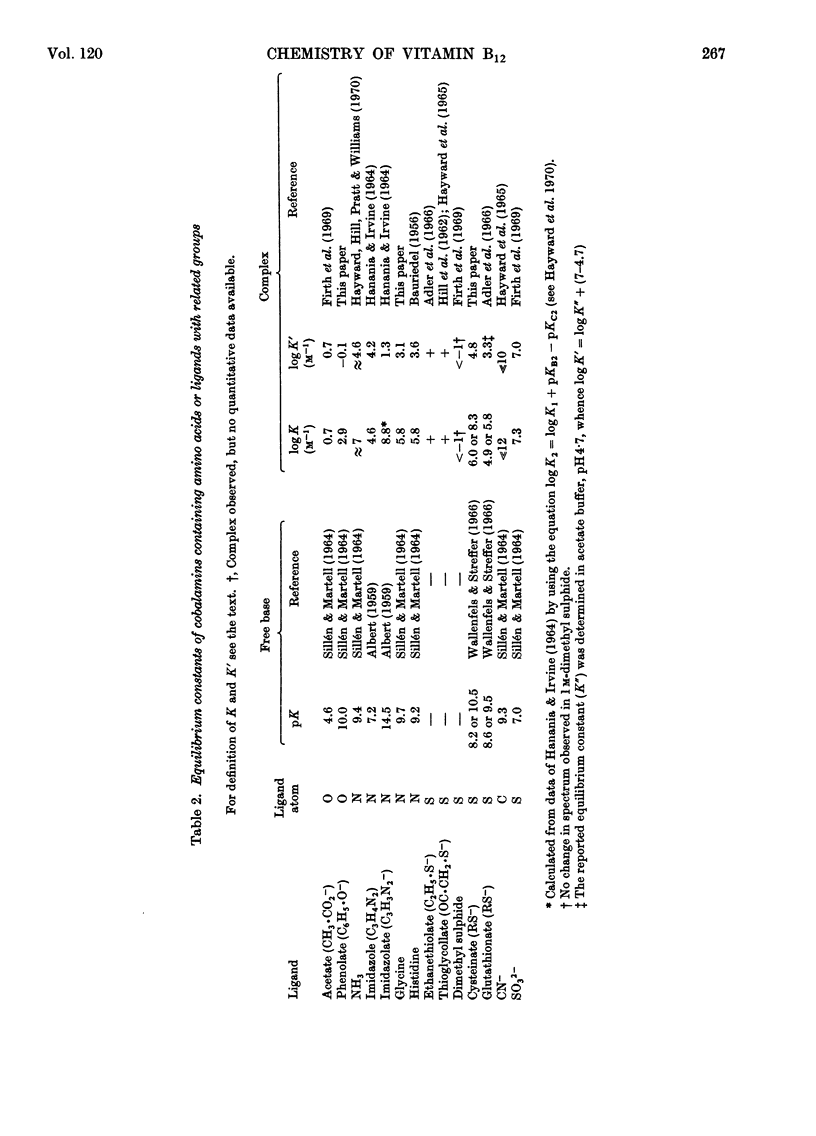
The following equilibrium constants (given as logK in units of m−1) were determined for the substitution of co-ordinated H2O in aquocobalamin by glycine (bound through N) 5.8, cysteine (bound through S) 6.0 or 8.3, depending on the value chosen for the pK of the thiol group, and phenolate 2.9. The spectrum of the phenolate cobalamin shows an additional intense absorption band at 468nm with a molar extinction coefficient of 1.1×104, which is assigned to a charge transfer from the phenolate to the cobalt ion. Equilibrium constants have also been determined for the equilibria between adenylcobamide cyanide and CN−, HO− and H+, which show that the adenine is more easily displaced by CN− and HO− than is 5,6-dimethylbenziminazole in vitamin B12, but can be protonated by acid while still remaining co-ordinated to the cobalt. It is shown that in the binding of corrinoids to proteins and polypeptides the formation of hydrogen bonds is far more important than co-ordination by the metal.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAURIEDEL W. R., PICKEN J. C., Jr, UNDERKOFLER L. A. Reactions of cyanocobalamin and aquocobalamin with proteins. Proc Soc Exp Biol Med. 1956 Mar;91(3):377–381. doi: 10.3181/00379727-91-22268. [DOI] [PubMed] [Google Scholar]
- BERNHAUER K., GAISER P., MUELLER O., WAGNER O. [On the chemistry and biochemistry of "cobalamine". 16. On corrinoid conjugates]. Biochem Z. 1960;333:106–110. [PubMed] [Google Scholar]
- Babior B. M., Kon H., Lecar H. The mechanism of action of ethanolamine deaminase. V. The photolysis of enzyme-bound alkylcobalamins. Biochemistry. 1969 Jun;8(6):2662–2669. doi: 10.1021/bi00834a063. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Li T. K. The binding of cobamides to ethanolamine deaminase. Biochemistry. 1969 Jan;8(1):154–160. doi: 10.1021/bi00829a022. [DOI] [PubMed] [Google Scholar]
- Firth R. A., Hill H. A., Pratt J. M., Thorp R. G. Separation and identification of organocobalt derivatives of vitamin B 12 on thin-layer cellulose. Anal Biochem. 1968 Jun;23(3):429–432. doi: 10.1016/0003-2697(68)90234-0. [DOI] [PubMed] [Google Scholar]
- GREGORY M. E., HOLDSWORTH E. S. Some properties of the cyanocobalamin-protein complex from sow's milk, and the mode of linkage of cyanocobalamin with protein. Biochem J. 1955 Feb;59(2):335–340. doi: 10.1042/bj0590335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDBOM A. A native cobalamin-polypeptide complex from liver: isolation and characterization. Biochem J. 1960 Feb;74:307–312. doi: 10.1042/bj0740307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. C., Hill H. A., Pratt J. M., Vanston N. J., Williams R. J. The chemistry of vitamin B 12. IV. The thermodynamic trans-effect. J Chem Soc Perkin 1. 1965 Sep;:6485–6493. [PubMed] [Google Scholar]
- PEEL J. L. THE CATALYSIS OF THE AUTO-OXIDATION OF 2-MERCAPTOETHANOL AND OTHER THIOLS BY VITAMIN B12 DERIVATIVES. POLAROGRAPHIC AND OTHER INVESTIGATIONS. Biochem J. 1963 Aug;88:296–308. doi: 10.1042/bj0880296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailes W. H., Hogenkamp H. P. The photolability of co-alkylcobinamides. Biochemistry. 1968 Dec;7(12):4160–4166. doi: 10.1021/bi00852a003. [DOI] [PubMed] [Google Scholar]
- WAGNER F., BERNHAUER K. NEW ASPECTS OF THE STRUCTURE OF CORRINOID COENZYMES. Ann N Y Acad Sci. 1964 Apr 24;112:580–589. doi: 10.1111/j.1749-6632.1964.tb45034.x. [DOI] [PubMed] [Google Scholar]
- WIJMENGA H. G., THOMPSON K. W., STERN K. G., O'CONNELL D. J. Preparation and properties of a cobalamin protein. Biochim Biophys Acta. 1954 Jan;13(1):144–146. doi: 10.1016/0006-3002(54)90287-5. [DOI] [PubMed] [Google Scholar]


