Abstract
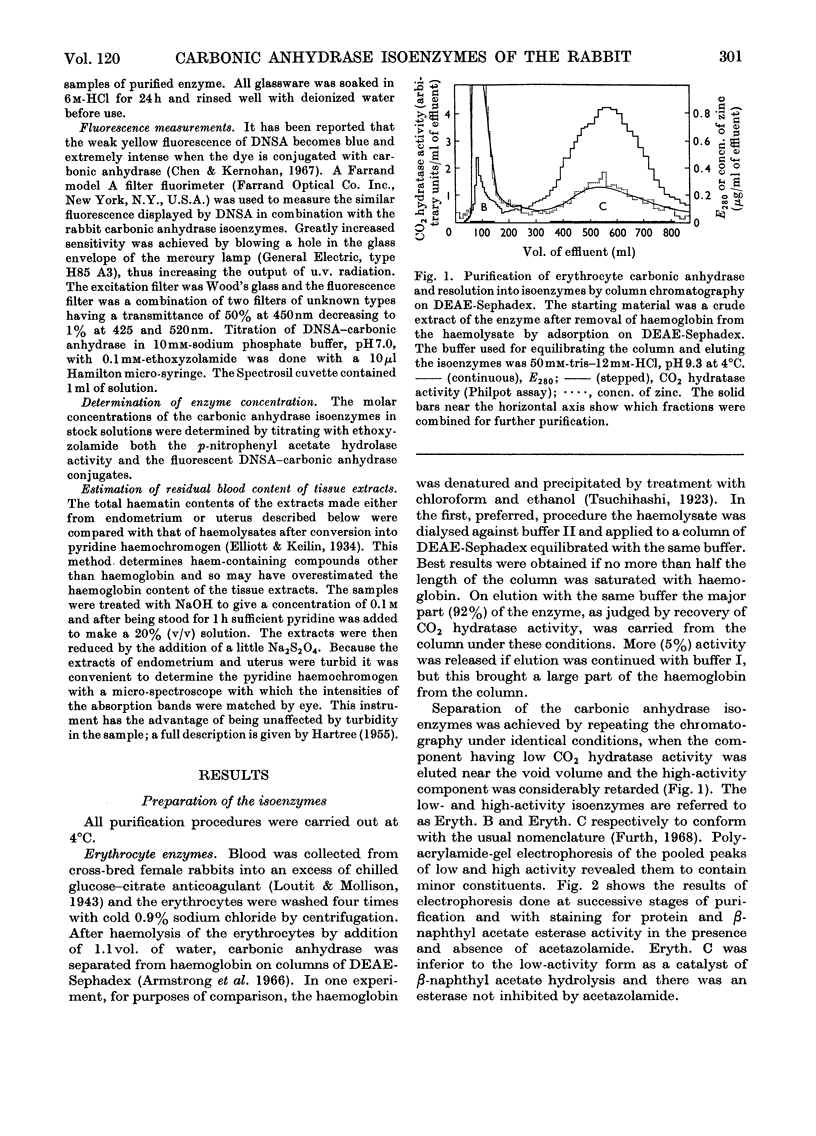
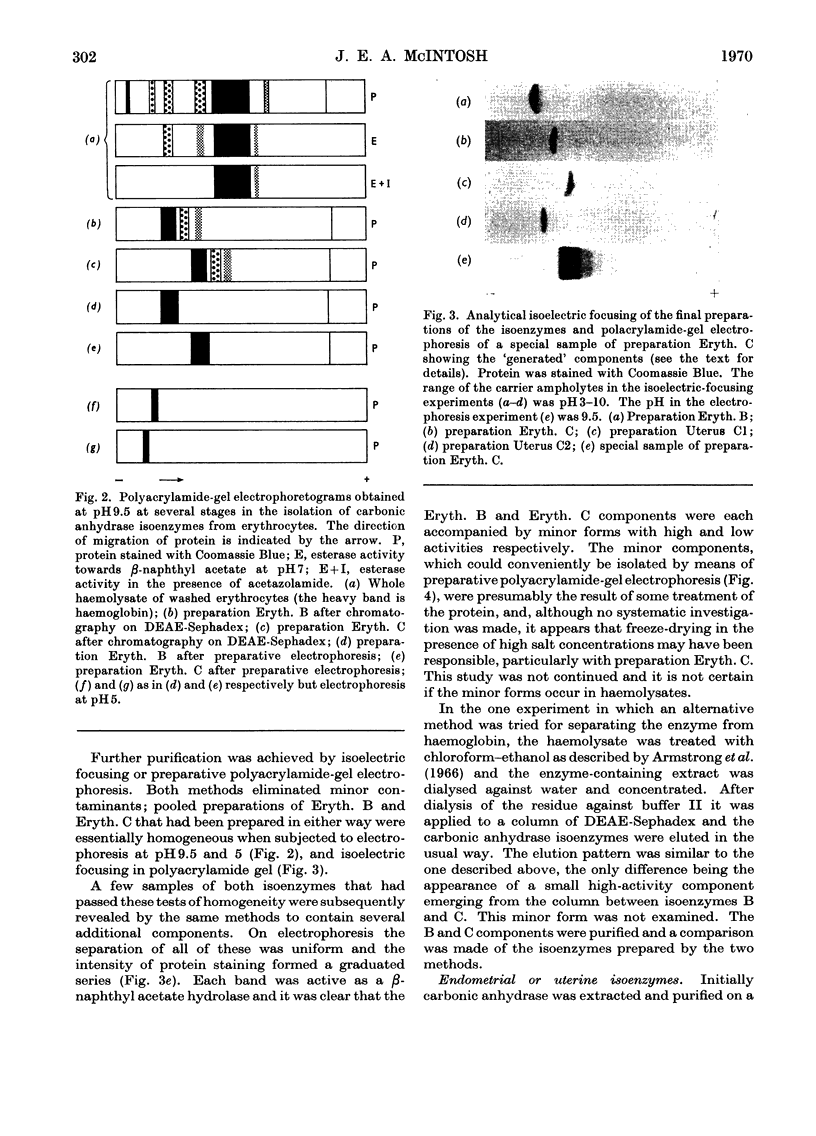
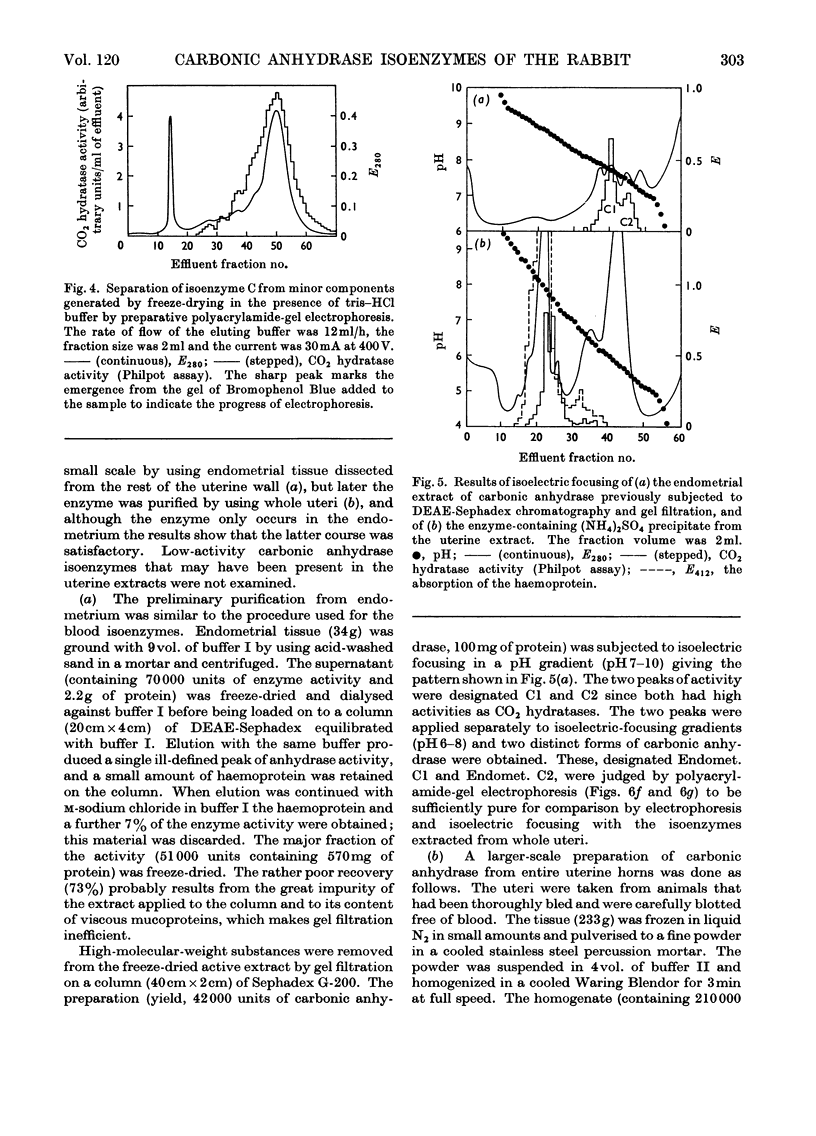
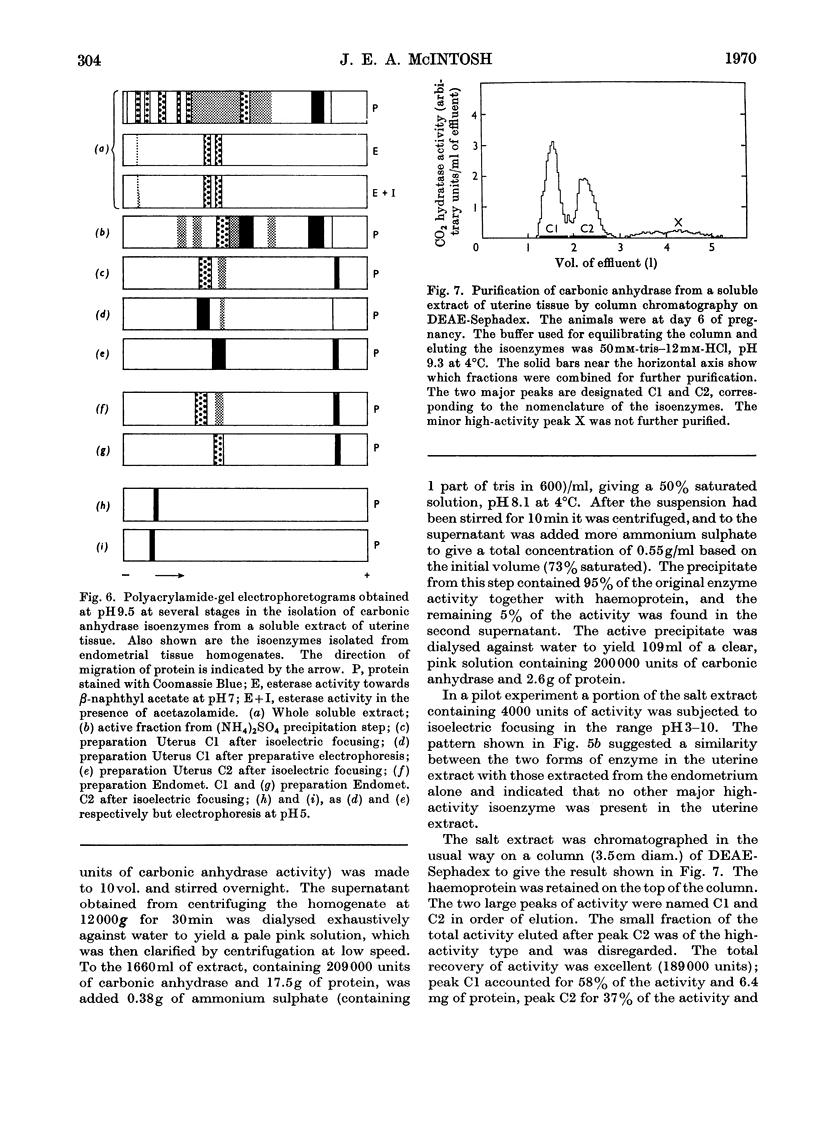
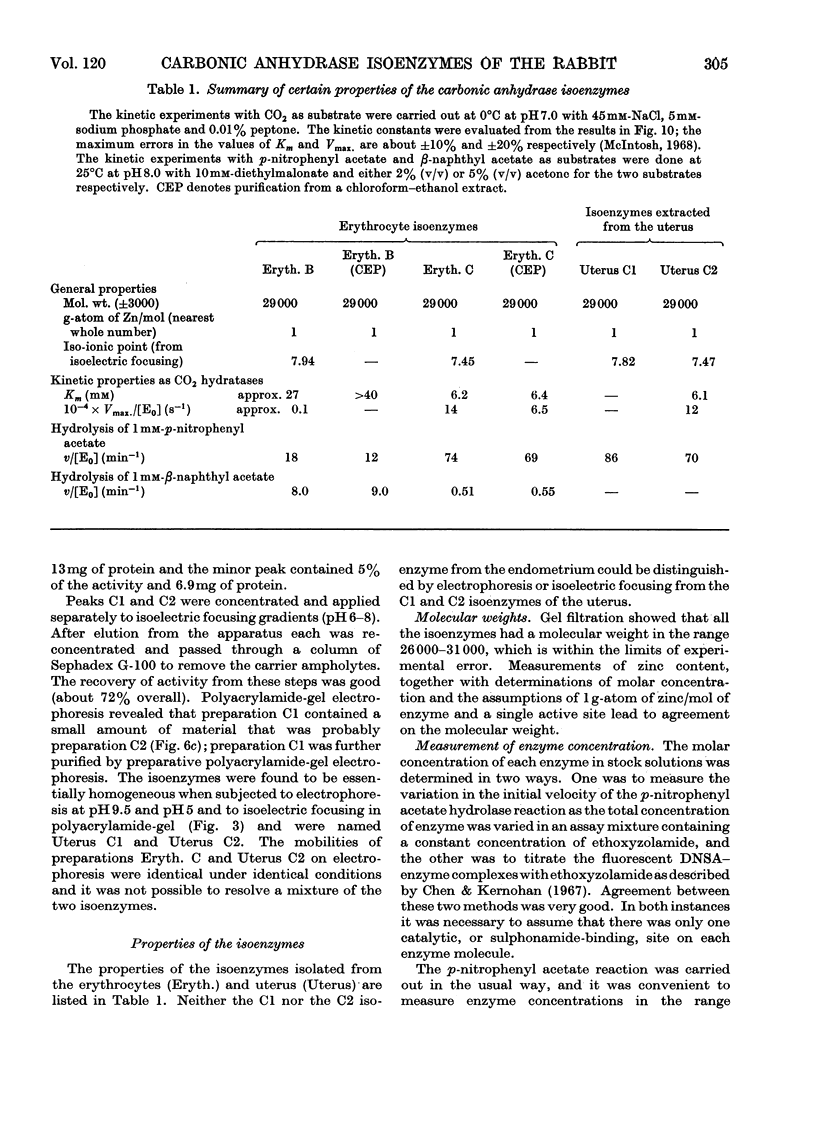
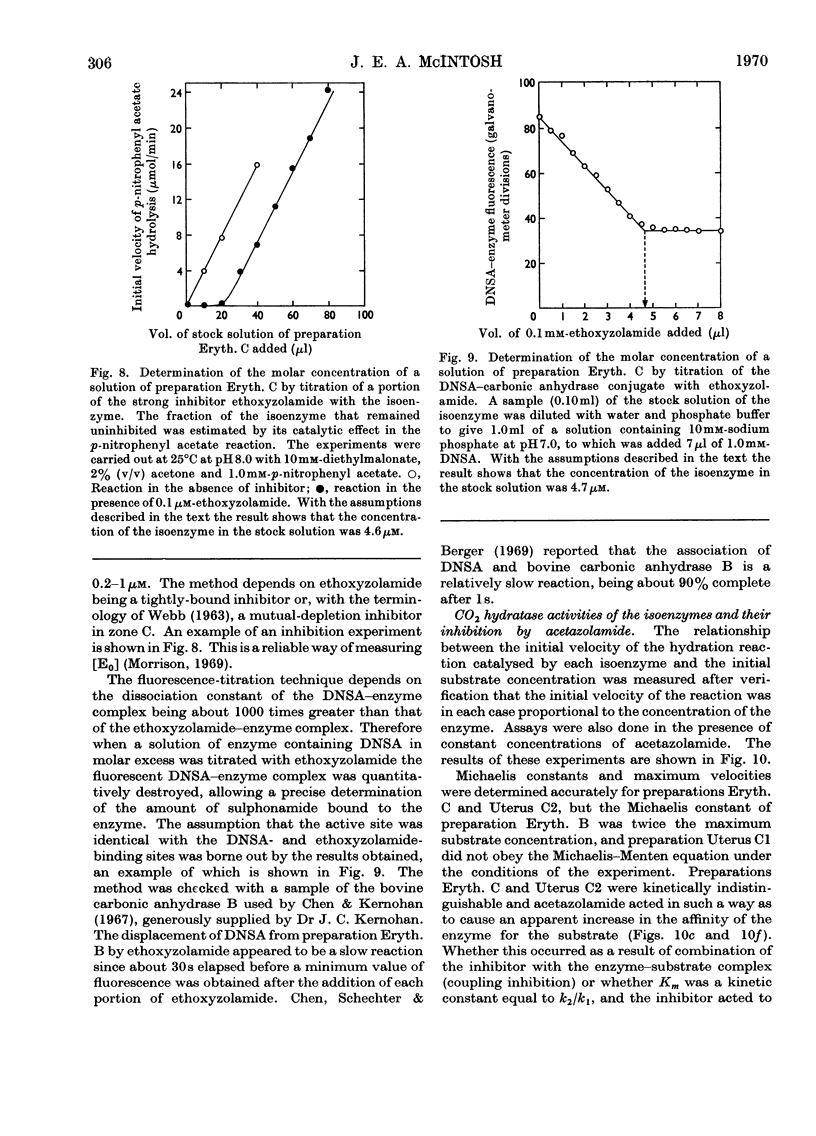
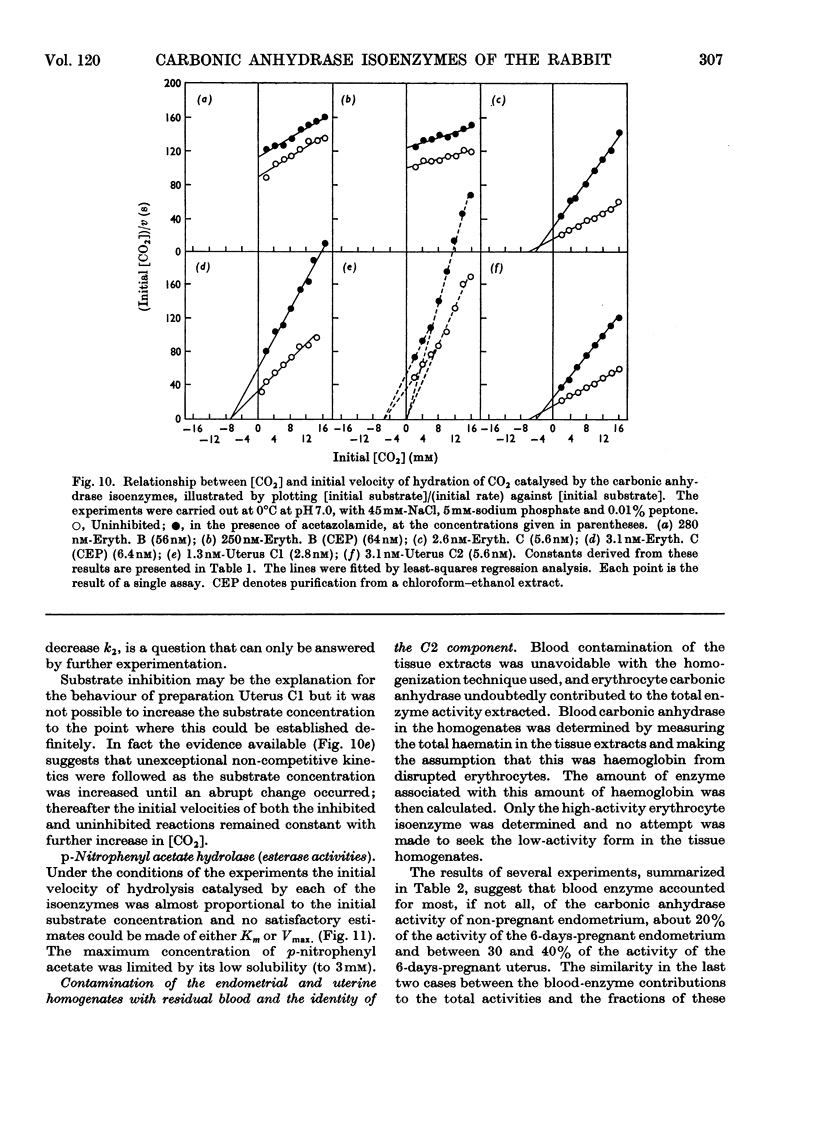
1. Two forms of the zinc-containing enzyme carbonic anhydrase (EC 4.2.1.1) were isolated from rabbit erythrocytes and two forms from rabbit uterine tissue (endometrium) in the progestational stage of pregnancy (days 6–8 of gestation). Separation of the isoenzymes was achieved by ion-exchange chromatography, preparative polyacrylamide-gel electrophoresis and isoelectric focusing. A comparison was made of the general properties and kinetic behaviour of the purified isoenzymes. 2. Although indistinguishable in terms of molecular weight and zinc content the isoenzymes were very different as catalysts of the hydration of carbon dioxide. The two erythrocyte isoenzymes, found in almost equal amounts, differed more than 100-fold in specific activity. Of the two isoenzymes prepared from either endometrial or entire uterine homogenates one was kinetically indistinguishable from the erythrocyte high-activity form, whereas the other, also possessing high activity, was found only in the endometrial or uterine tissue. Present evidence suggests that the former isoenzyme originated from residual blood contaminating the tissue homogenates, and that a marked rise in the content of the latter isoenzyme accounts for the increase in rabbit endometrial carbonic anhydrase activity that previously has been observed in early pregnancy. 3. Minor forms of the erythrocyte isoenzymes, having a characteristic quantitative and electrophoretic relationship to one another, were occasionally produced during purification. 4. The actions were investigated of the inhibitors acetazolamide (5-acetamido-3,4-diazole-1-thia-2-sulphonamide), 1,1-dimethylaminonaphthalene-5-sulphonamide and ethoxyzolamide (6-ethoxybenzothiazole-2-sulphonamide) on the hydration of carbon dioxide and the hydrolysis of p-nitrophenyl acetate catalysed by the isoenzymes. 5. The low-activity erythrocyte isoenzyme was superior to the high-activity form as a catalyst of β-naphthyl acetate hydrolysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F., Kernohan J. C. Combination of bovine carbonic anhydrase with a fluorescent sulfonamide. J Biol Chem. 1967 Dec 25;242(24):5813–5823. [PubMed] [Google Scholar]
- Chen R. F., Schechter A. N., Berger R. L. Stopped-flow fluorometry with available instrumentation. Anal Biochem. 1969 Apr 11;29(1):68–75. doi: 10.1016/0003-2697(69)90008-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Duff T. A., Coleman J. E. Macaca mulata carbonic anhydrase. Crystallization and physicochemical and enzymatic properties of two isozymes. Biochemistry. 1966 Jun;5(6):2009–2019. doi: 10.1021/bi00870a032. [DOI] [PubMed] [Google Scholar]
- Funakoshi S., Deutsch H. F. Human carbonic anhydrases. I. Isolation and demonstration of isozymes in erythrocytes. J Biol Chem. 1968 Dec 25;243(24):6474–6481. [PubMed] [Google Scholar]
- Funakoshi S., Deutsch H. F. Human carbonic anhydrases. II. Some physicochemical properties of native isozymes and of similar isozymes generated in vitro. J Biol Chem. 1969 Jul 10;244(13):3438–3446. [PubMed] [Google Scholar]
- Furth A. J. Purification and properties of horse erythrocyte carbonic anhydrases. J Biol Chem. 1968 Sep 25;243(18):4832–4841. [PubMed] [Google Scholar]
- LINDSKOG S. Purification and properties of bovine erythrocyte carbonic anhydrase. Biochim Biophys Acta. 1960 Apr 8;39:218–226. doi: 10.1016/0006-3002(60)90156-6. [DOI] [PubMed] [Google Scholar]
- LUTWAK-MANN C., ADAMS C. E. Carbonic anhydrase in the female reproductive tract. II. Endometrial carbonic anhydrase as indicator of luteoid potency: correlation with progestational proliferation. J Endocrinol. 1957 Apr;15(1):43–55. doi: 10.1677/joe.0.0150043. [DOI] [PubMed] [Google Scholar]
- LUTWAK-MANN C. Carbonic anhydrase in the female reproductive tract; occurrence, distribution and hormonal dependence. J Endocrinol. 1955 Oct;13(1):26–38. doi: 10.1677/joe.0.0130026. [DOI] [PubMed] [Google Scholar]
- LUTWAK-MANN C. The occurrence of carbonic anhydrase in the rabbit uterus. J Endocrinol. 1954 Nov;11(4):xi–xii. [PubMed] [Google Scholar]
- Lutwak-Mann C., McIntosh J. E. Zinc and carbonic anhydrase in the rabbit uterus. Nature. 1969 Mar 22;221(5186):1111–1114. doi: 10.1038/2211111a0. [DOI] [PubMed] [Google Scholar]
- McIntosh J. E. Assay of carbonic anhydrase by titration at constant pH. Biochem J. 1968 Sep;109(2):203–207. doi: 10.1042/bj1090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J. E. Carbonic anhydrase isoenzymes in the erythrocytes and dorsolateral prostate of the rat. Biochem J. 1969 Sep;114(3):463–476. doi: 10.1042/bj1140463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- NYMAN P. O. Purification and properties of carbonic anhydrase from human erythrocytes. Biochim Biophys Acta. 1961 Sep 2;52:1–12. doi: 10.1016/0006-3002(61)90898-8. [DOI] [PubMed] [Google Scholar]
- Philpot F. J., Philpot J. S. A modified colorimetric estimation of carbonic anhydrase. Biochem J. 1936 Dec;30(12):2191–2193. doi: 10.1042/bj0302191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocker Y., Dickerson D. G. The catalytic versatility of erythrocyte carbonic anhydrase. V. Kinetic studies of enzyme-catalyzed hydrations of aliphatic aldehydes. Biochemistry. 1968 May;7(5):1995–2004. doi: 10.1021/bi00845a050. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. The enzyme-catalyzed hydrolysis of rho-nitrophenyl acetate. J Am Chem Soc. 1965 Dec 5;87(23):5497–5498. doi: 10.1021/ja00951a049. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. VI. Kinetic studies of noncompetitive inhibition of enzyme-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1968 Aug;7(8):2936–2945. doi: 10.1021/bi00848a034. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- TASHIAN R. E., SHAW M. W. Inheritance of an erythrocyte acetylesterase variant in man. Am J Hum Genet. 1962 Sep;14:295–300. [PMC free article] [PubMed] [Google Scholar]
- Tashian R. E., Shreffler D. C., Shows T. B. Genetic and phylogenetic variation in the different molecular forms of mammalian erythrocyte carbonic anhydrases. Ann N Y Acad Sci. 1968 Jun 14;151(1):64–77. doi: 10.1111/j.1749-6632.1968.tb11878.x. [DOI] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. II. Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem J. 1952 May;51(2):155–167. doi: 10.1042/bj0510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. L., Fölsch G., Nyman P. O., Malmström B. G. Inhibition of human erythrocyte carbonic anhydrase B by chloroacetyl sulfonamides with labeling of the active site. J Biol Chem. 1967 Sep 25;242(18):4206–4211. [PubMed] [Google Scholar]