Abstract
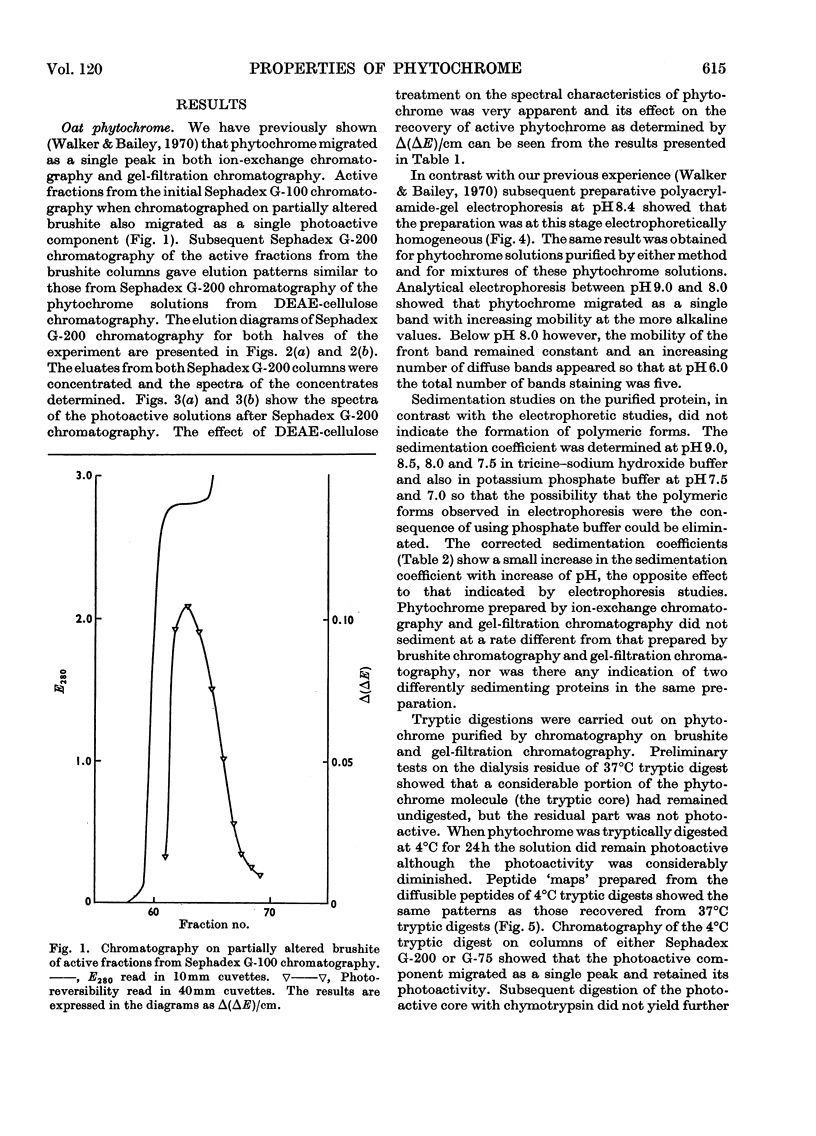
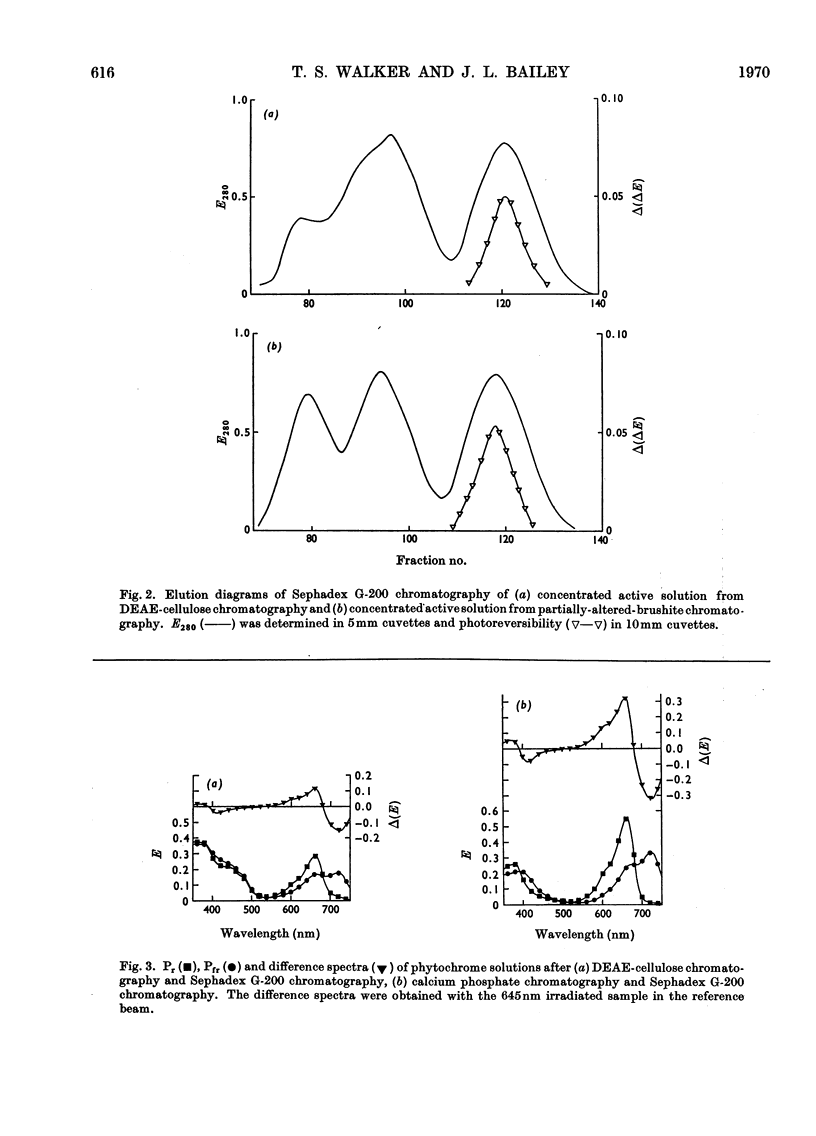
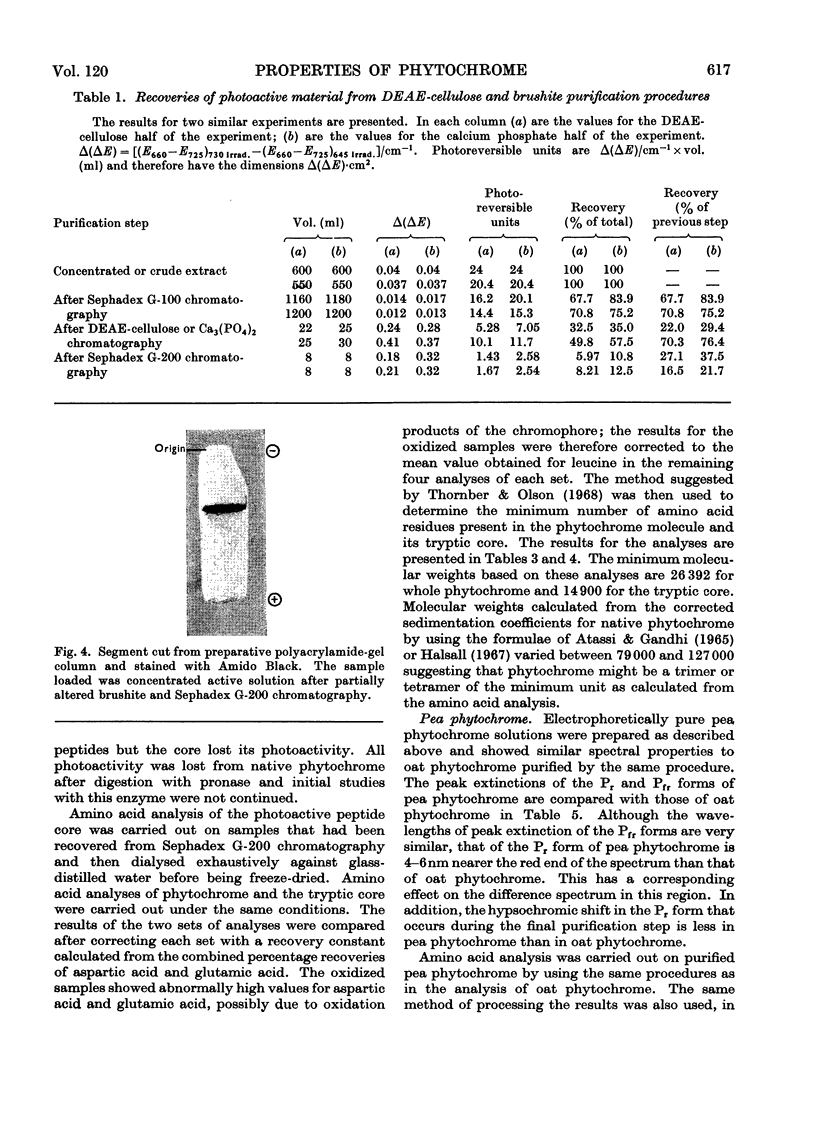
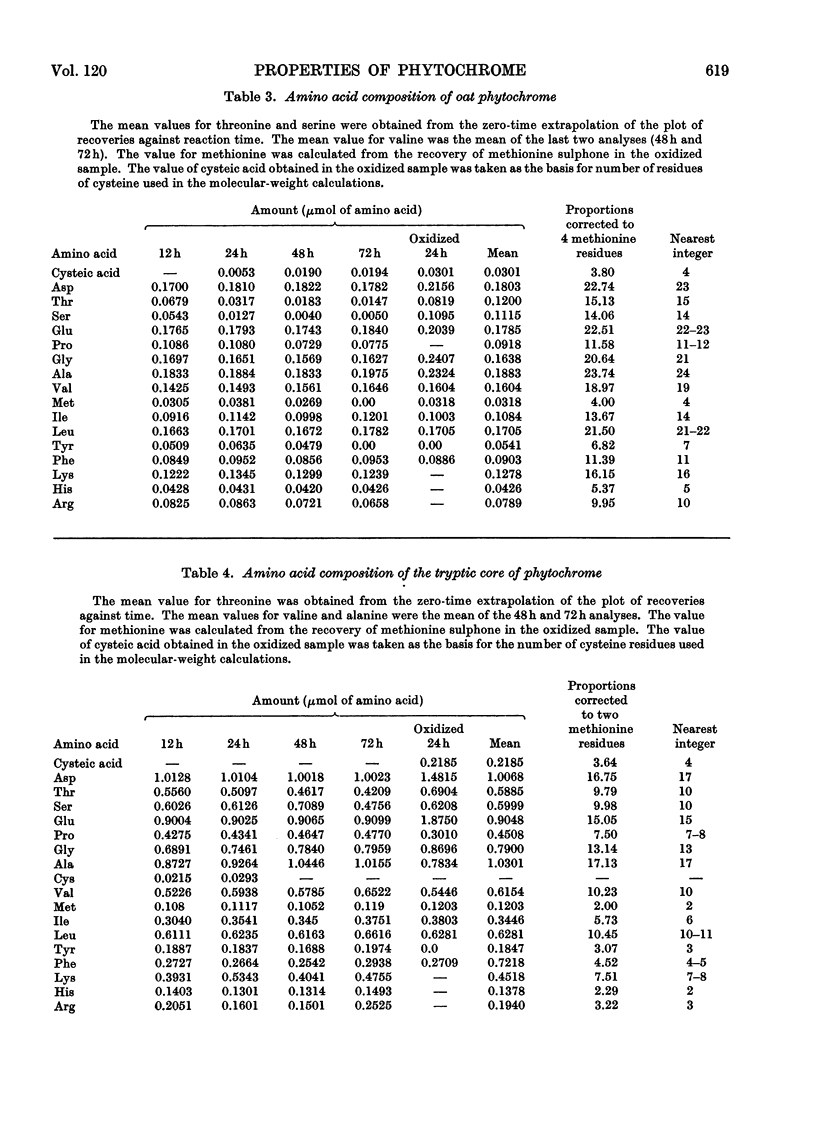
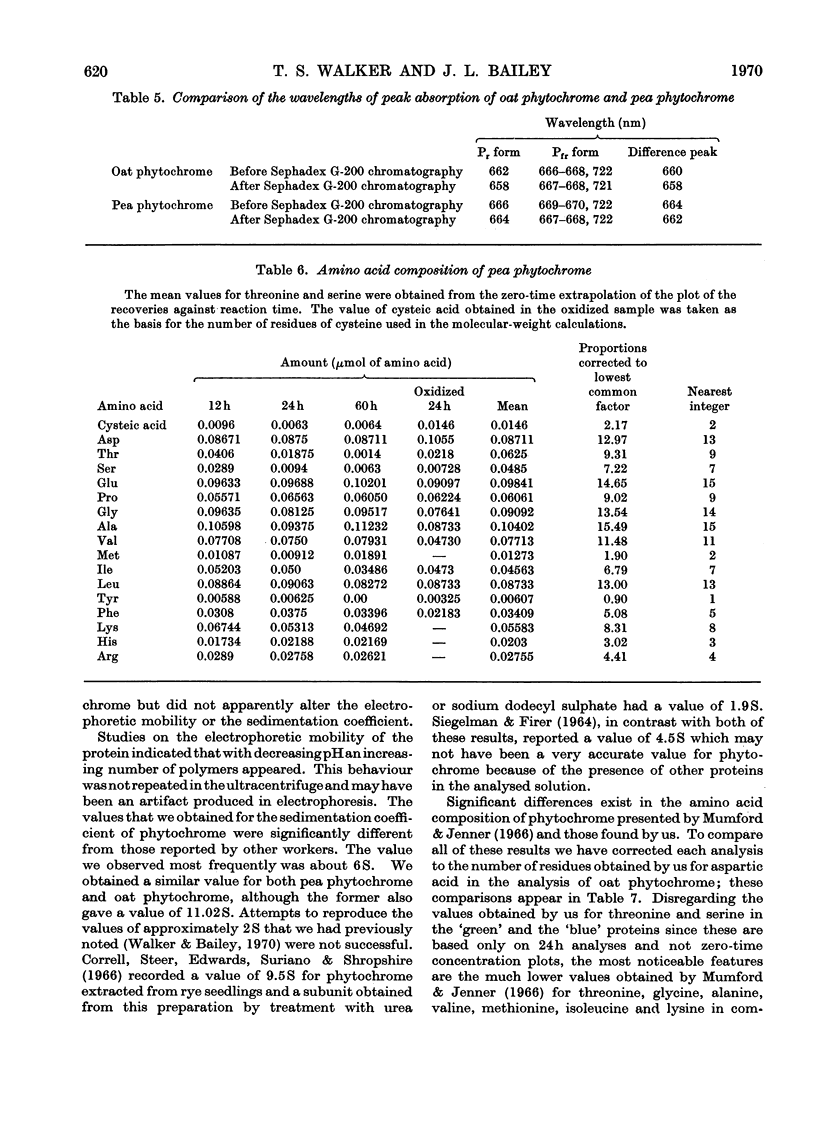
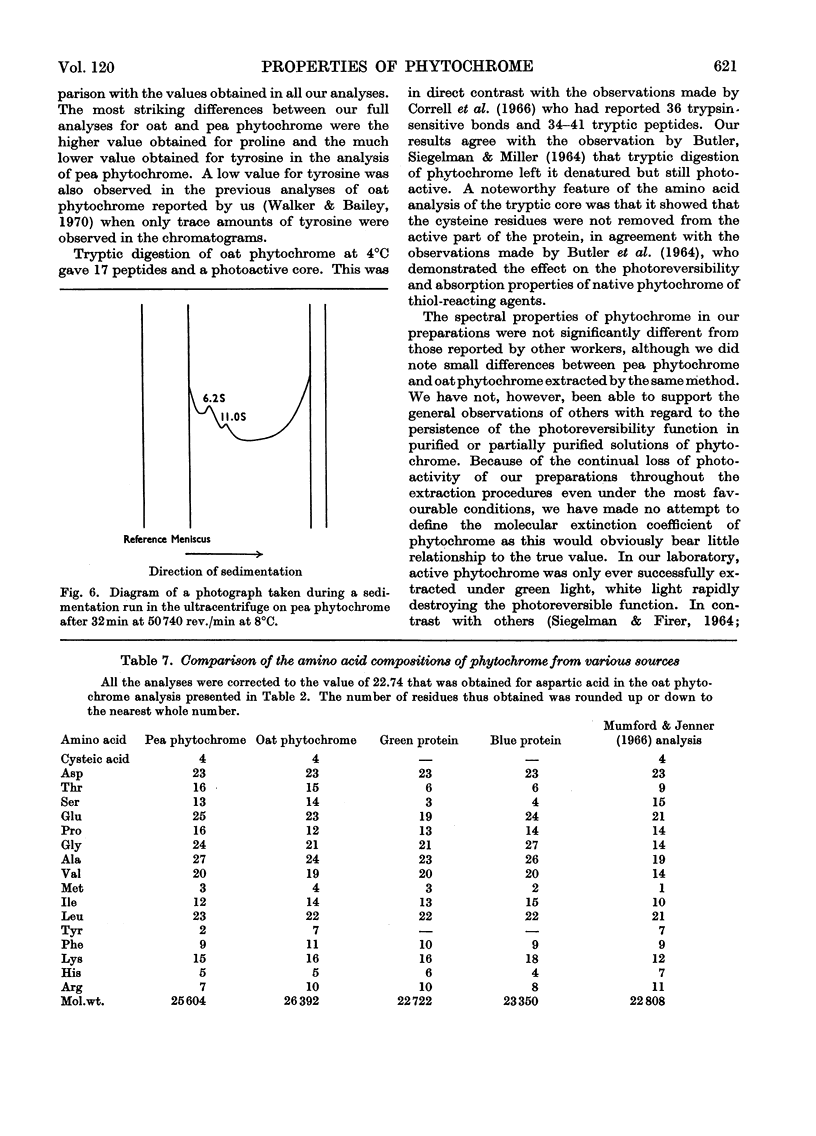
1. Phytochrome was purified from etiolated oat (Avena sativa) seedlings either by gel-filtration chromatography and ion-exchange chromatography or by gel-filtration chromatography and calcium phosphate chromatography. Differences were observed in the spectral properties of phytochrome isolated by the two methods. 2. Electrophoresis of pure phytochrome at pH values between 9.0 and 6.0 showed the tendency of phytochrome to form different molecular species. Studies in the ultracentrifuge did not show a corresponding change in the sedimentation coefficient with the change in pH. 3. Tryptic digestion of electrophoretically pure phytochrome gave 17 peptides and a photoactive core. The amino acid composition of the core is reported and compared with the analysis of whole phytochrome. 4. Some properties of phytochrome isolated from Pisum sativum are compared with those of phytochrome from A. sativa. 5. The properties of phytochrome purified by other workers are compared with our findings.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATASSI M. Z., GANDHI S. K. AN EMPIRICAL FORMULA FOR CALCULATING MOLECULAR WEIGHTS OF PROTEINS. Naturwissenschaften. 1965 May;52:259–259. doi: 10.1007/BF00602925. [DOI] [PubMed] [Google Scholar]
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Zollinger W. D., Platz B. B. Some Properties of Phytochrome Isolated From Dark-grown Oat Seedlings (Avena sativa L.). Plant Physiol. 1968 Aug;43(8):1239–1243. doi: 10.1104/pp.43.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hillman W. S. Rapid Destruction of the P(FS) Form of Phytochrome by a Substance in Extracts of Pisum Tissue. Plant Physiol. 1966 Sep;41(7):1242–1244. doi: 10.1104/pp.41.7.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD N. E. Uncoupling of the Hill reaction from photophosphorylation by anions. Arch Biochem Biophys. 1962 Mar;96:653–661. doi: 10.1016/0003-9861(62)90352-1. [DOI] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Purification and characterization of phytochrome from oat seedlings. Biochemistry. 1966 Nov;5(11):3657–3662. doi: 10.1021/bi00875a039. [DOI] [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Wieczorek G. A., Turner B. C. Preparation of calcium phosphate for protein chromatography. Anal Biochem. 1965 Dec;13(3):402–404. doi: 10.1016/0003-2697(65)90332-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. O., Bonner B. A. Isolation of phytochrome from the alga mesotaenium and liverwort sphaerocarpos. Plant Physiol. 1967 Jun;42(6):762–766. doi: 10.1104/pp.42.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Olson J. M. The chemical composition of a crystalline bacteriochlorophyll-protein complex isolated from the green bacterium, Chloropseudomonas ethylicum. Biochemistry. 1968 Jun;7(6):2242–2249. doi: 10.1021/bi00846a029. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Bailey J. L. Studies on phytochrome. Two photoreversible chromoproteins from etiolated oat seedlings. Biochem J. 1970 Dec;120(3):607–612. doi: 10.1042/bj1200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Bailey J. L. Two spectrally different forms of the phytochrome chromophore extracted from etiolated oat seedlings. Biochem J. 1968 Apr;107(4):603–605. doi: 10.1042/bj1070603. [DOI] [PMC free article] [PubMed] [Google Scholar]



