Abstract
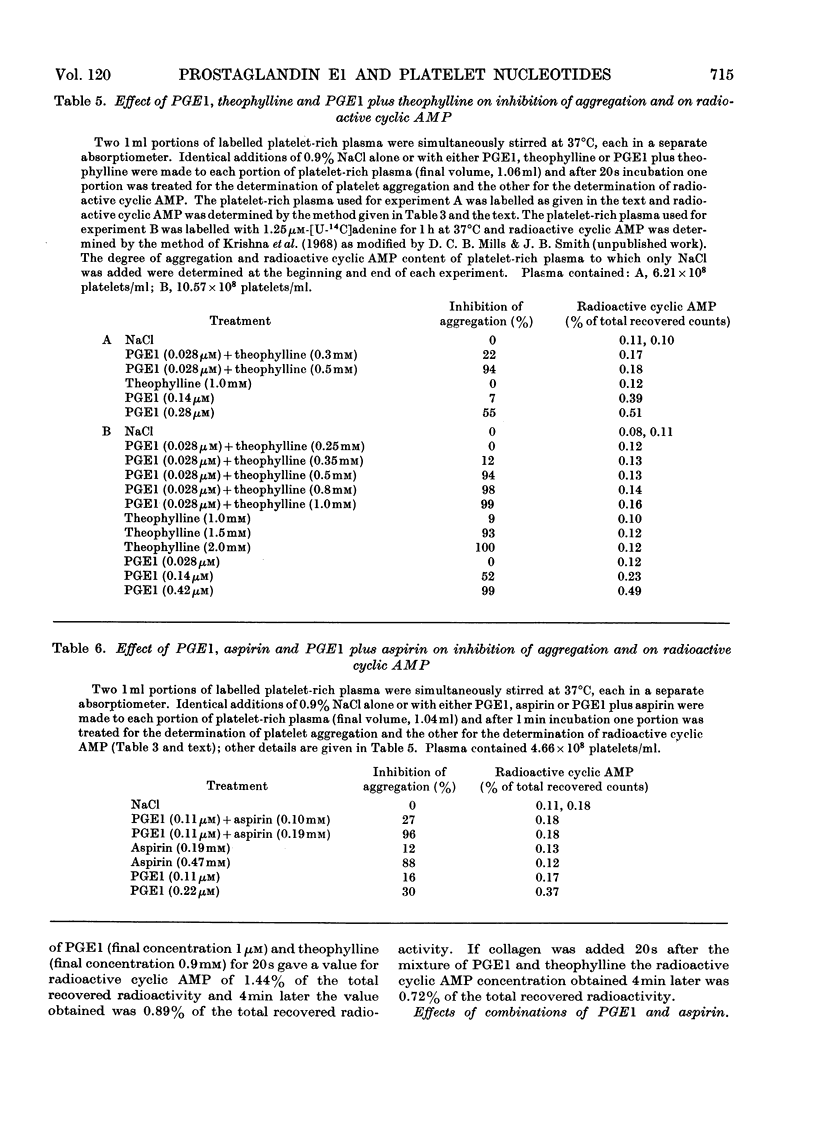
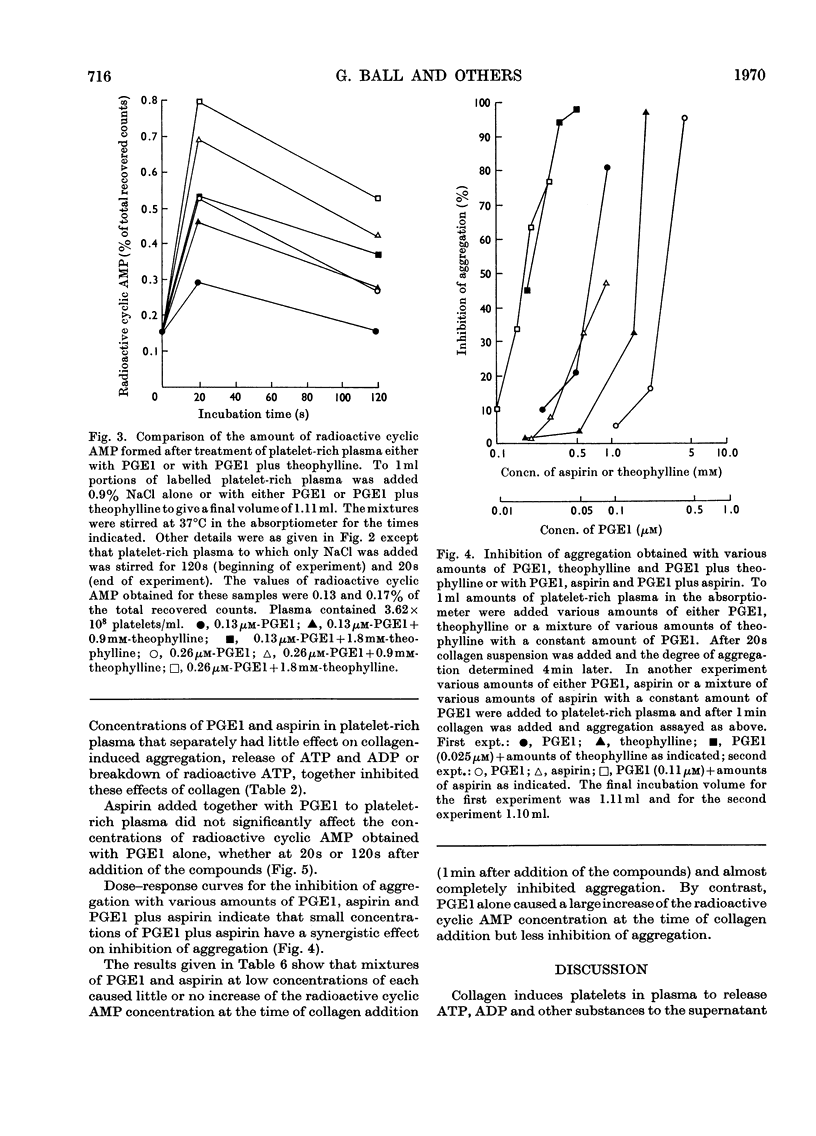
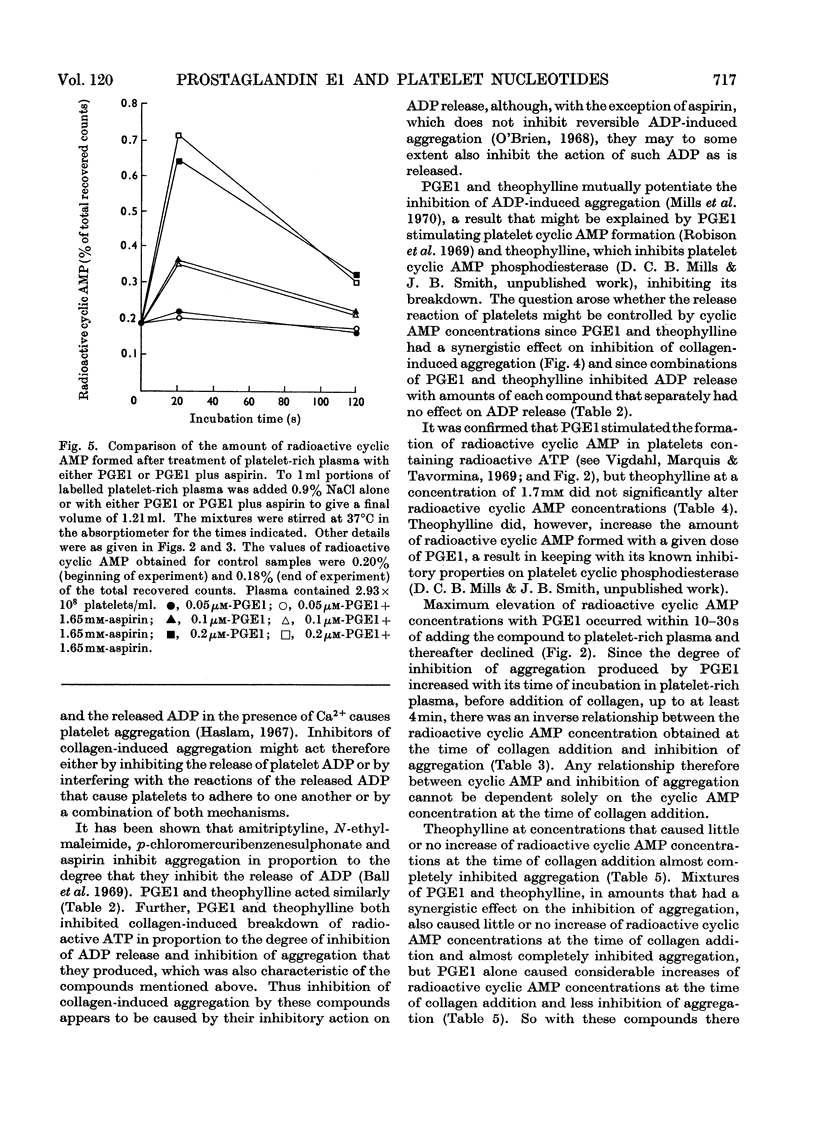
1. Human platelet nucleotides were labelled by incubating platelet-rich plasma with [U-14C]adenine. With such platelets, the effects of prostaglandin E1, theophylline and aspirin were determined on collagen-induced platelet aggregation and release of platelet ATP and ADP. Intracellular changes of platelet radioactive nucleotides, particularly 3′:5′-cyclic AMP, were also determined both with and without collagen treatment. 2. Prostaglandin E1, theophylline and aspirin inhibited collagen-induced aggregation of platelets in a dose-dependent manner. Collagen-induced release of ATP and ADP and breakdown of radioactive ATP were also inhibited in a dose-dependent manner. 3. Prostaglandin E1 stimulated the formation of platelet radioactive 3′:5′-cyclic AMP in a dose-dependent manner. With a given dose of prostaglandin E1, maximum formation of radioactive 3′:5′-cyclic AMP occurred by 10–30s and thereafter the concentrations declined. The degree of inhibition of aggregation produced by prostaglandin E1, however, increased with its time of incubation in platelet-rich plasma before addition of collagen, so that there was an inverse relationship between the radioactive 3′:5′-cyclic AMP concentration measured at the time of collagen addition and the subsequent degree of inhibition of aggregation obtained. 4. Neither theophylline nor aspirin at a concentration in platelet-rich plasma of 1.7mm altered platelet radioactive 3′:5′-cyclic AMP contents. In the presence of prostaglandin E1, theophylline increased the concentration of radioactive 3′:5′-cyclic AMP over that noted with prostaglandin E1 alone, but aspirin did not. 5. Mixtures of prostaglandin E1 and theophylline had a synergistic effect on inhibition of platelet aggregation. The same was true to a lesser extent with mixtures of prostaglandin E1 and aspirin. Such mixtures also inhibited collagen-induced release of platelet ATP and ADP and breakdown of platelet radioactive ATP. 6. Certain concentrations of either theophylline or aspirin and mixtures of small concentrations of prostaglandin E1 with either theophylline or aspirin caused little or no increase of radioactive 3′:5′-cyclic AMP at the time of collagen addition, but inhibited aggregation to a marked degree, whereas higher concentrations of prostaglandin E1 alone caused a much greater increase of radioactive 3′:5′-cyclic AMP at the time of collagen addition but inhibited aggregation to a lesser extent. With these compounds there does not appear to be a correlation between these parameters.
Full text
PDF

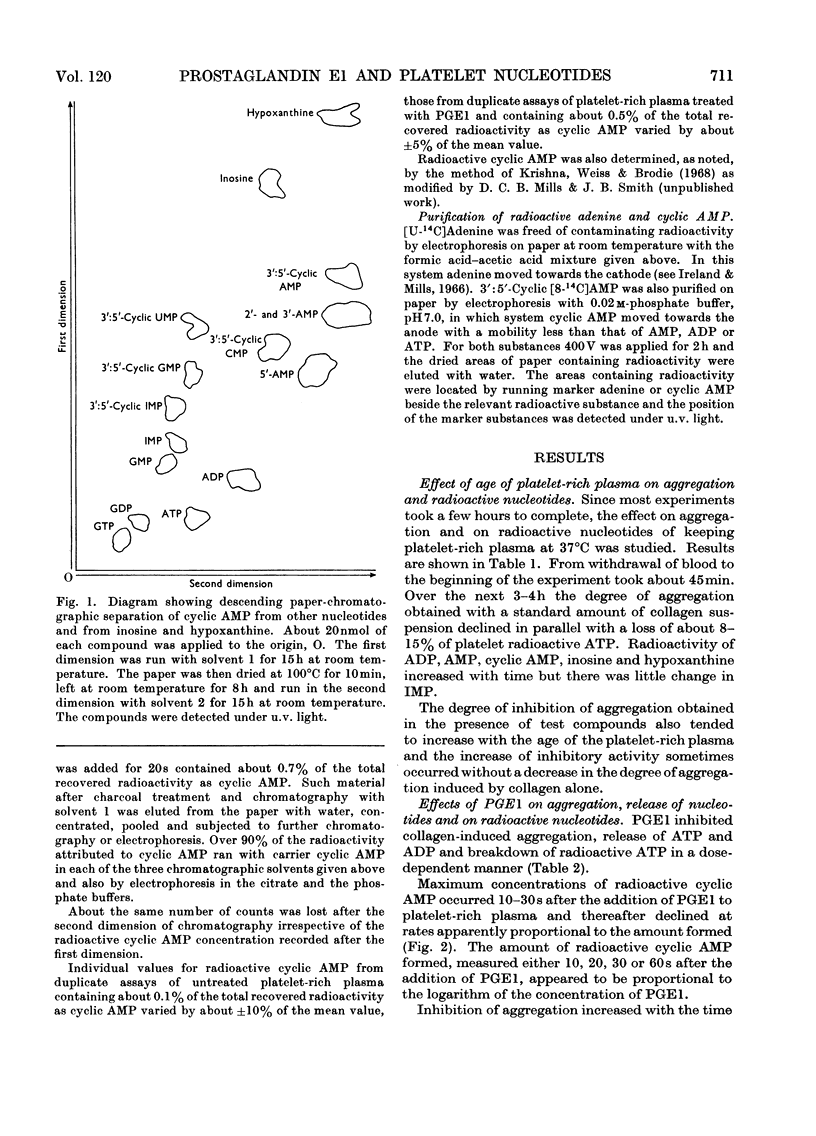
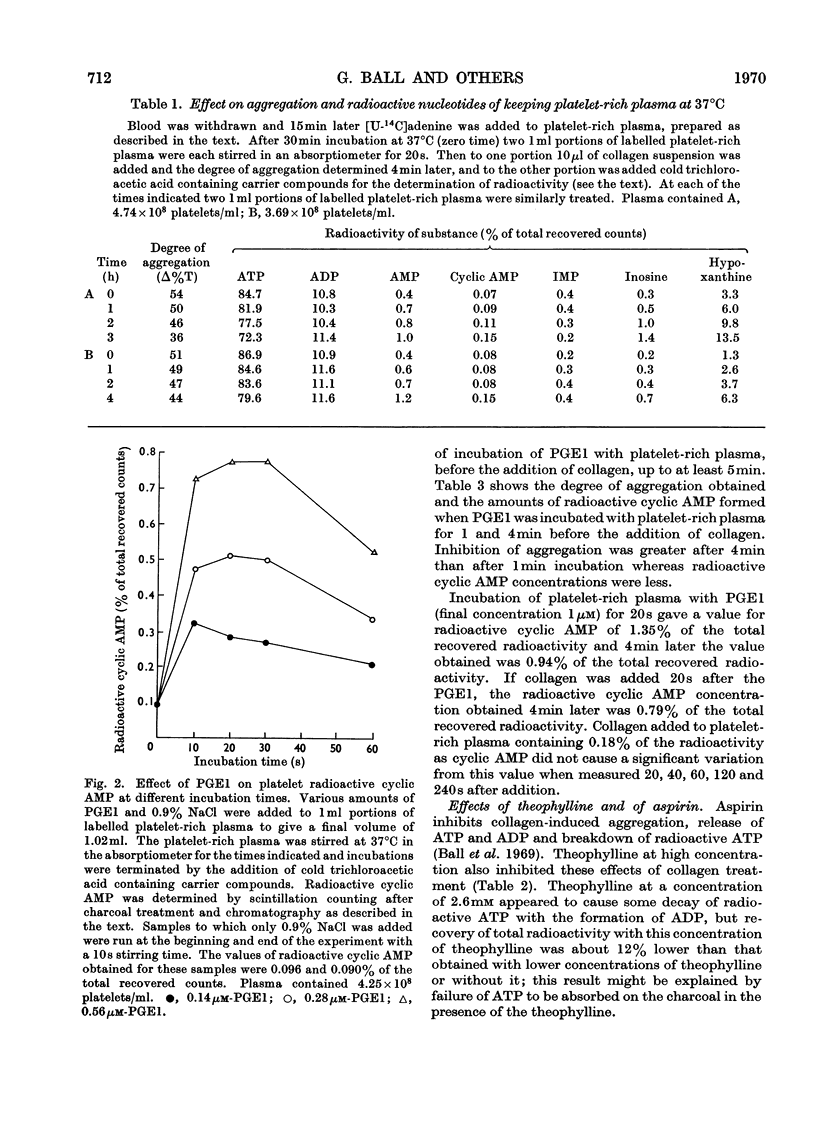
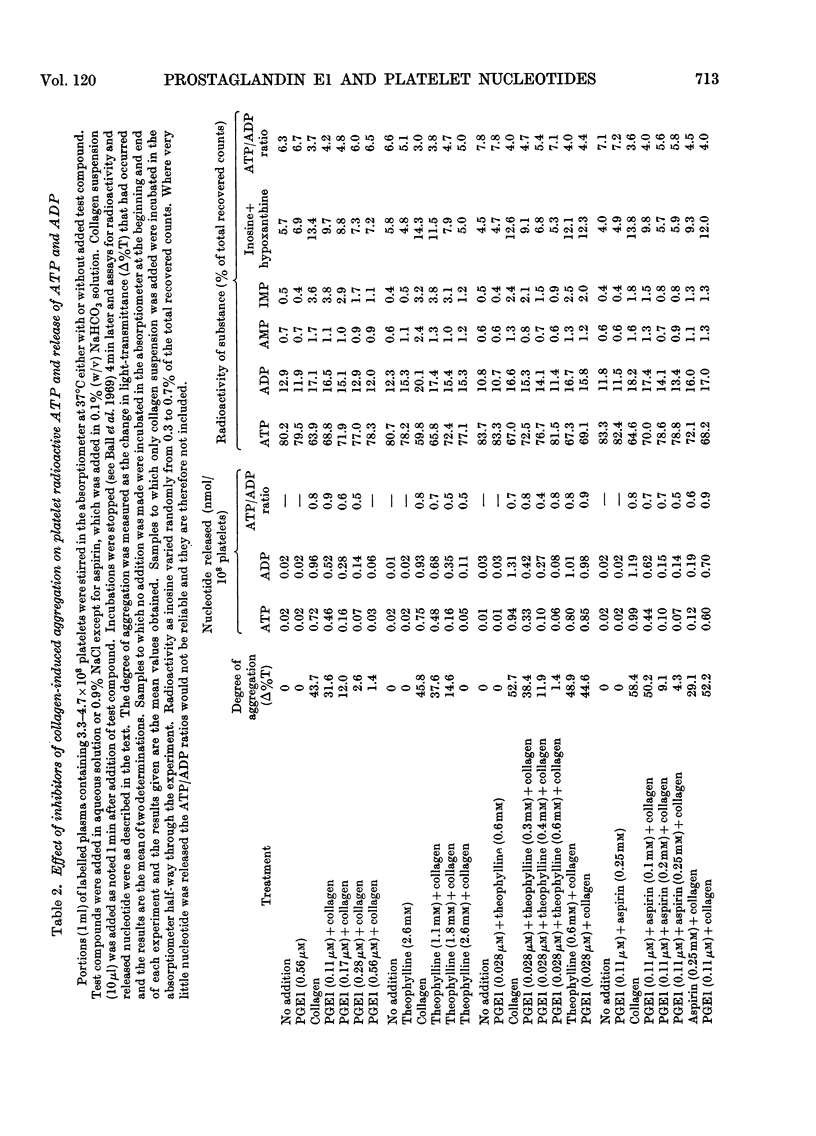
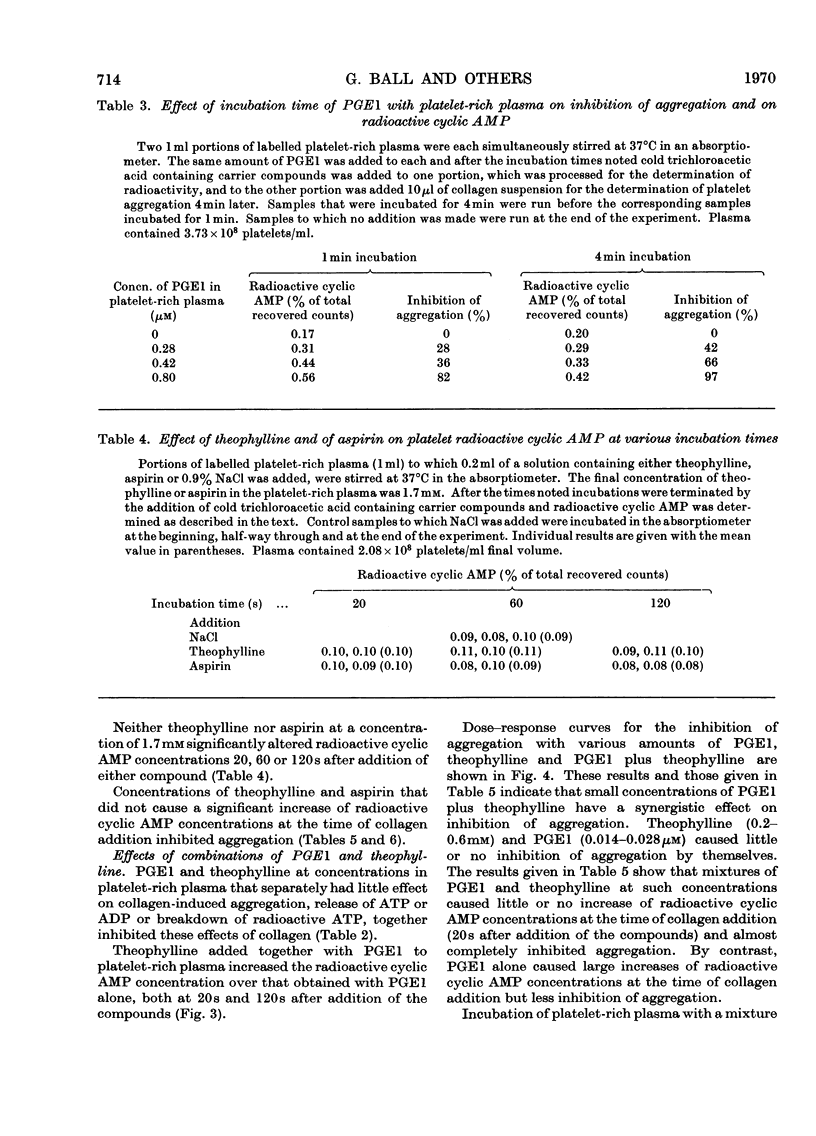

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardlie N. G., Glew G., Schultz B. G., Schwartz C. J. Inhibition and reversal of platelet aggregation by methyl xanthines. Thromb Diath Haemorrh. 1967 Dec 31;18(3-4):670–673. [PubMed] [Google Scholar]
- Ball G., Fulwood M., Ireland D. M., Yates P. Effect of some inhibitors of platelet aggregation on platelet nucleotides. Biochem J. 1969 Sep;114(3):669–671. doi: 10.1042/bj1140669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons P. R., Hampton J. R., Harrison M. J., Honour A. J., Mitchell J. R. Effect of prostaglandin E1 on platelet behaviour in vitro and in vivo. Br Med J. 1967 May 20;2(5550):468–472. doi: 10.1136/bmj.2.5550.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G., Packham M. A., Nishizawa E. E., Mustard J. F., Murphy E. A. The effect of acetylsalicyclic acid on platelet function. J Exp Med. 1968 Nov 1;128(5):877–894. doi: 10.1084/jem.128.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Holmsen I., Bernhardsen A. Microdetermination of adenosine diphosphate and adenosine triphosphate in plasma with firefly luciferase system. Anal Biochem. 1966 Dec;17(3):456–473. doi: 10.1016/0003-2697(66)90181-3. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Rounbehler M., Kuehl F. A., Jr A new assay for measuring adenyl cyclase activity in intact cells. Anal Biochem. 1969 Nov;32(2):210–217. doi: 10.1016/0003-2697(69)90077-3. [DOI] [PubMed] [Google Scholar]
- Ireland D. M., Mills D. C. Detection and determination of adenosine diphosphate and related substances in plasma. Biochem J. 1966 May;99(2):283–296. doi: 10.1042/bj0990283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Marquis N. R., Vigdahl R. L., Tavormina P. A. Platelet aggregation. I. Regulation by cyclic AMP and prostaglandin E1. Biochem Biophys Res Commun. 1969 Sep 10;36(6):965–972. doi: 10.1016/0006-291x(69)90298-8. [DOI] [PubMed] [Google Scholar]
- Mills D. C., Roberts G. C. Effects of adrenaline on human blood platelets. J Physiol. 1967 Nov;193(2):443–453. doi: 10.1113/jphysiol.1967.sp008369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. R. Effects of salicylates on human platelets. Lancet. 1968 Apr 13;1(7546):779–783. doi: 10.1016/s0140-6736(68)92228-9. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Neri L. L. Cyclic 3',5'-adenosine monophosphate in human blood platelets. Nature. 1969 Nov 8;224(5219):609–610. doi: 10.1038/224609a0. [DOI] [PubMed] [Google Scholar]
- Vigdahl R. L., Marquis N. R., Tavormina P. A. Platelet aggregation. II. Adenyl cyclase, prostaglandin E1, and calcium. Biochem Biophys Res Commun. 1969 Oct 22;37(3):409–415. doi: 10.1016/0006-291x(69)90930-9. [DOI] [PubMed] [Google Scholar]


